Compounds
This lesson covers:
- What a compound is
- How chemical reactions form compounds
- Properties of compounds and their elements
What is a compound?
Atoms can join together to form molecules. The join between the atoms is a chemical bond.
When atoms from two or more different elements bond together, they form a compound.
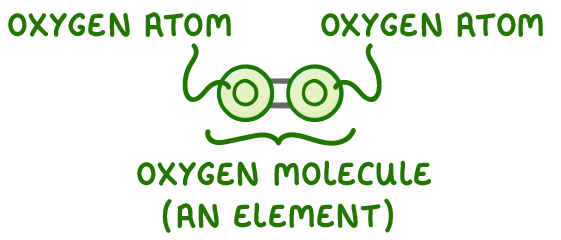
Oxygen atoms join to form a molecule of oxygen.
The atoms that are chemically joined are the same, so it’s an element.
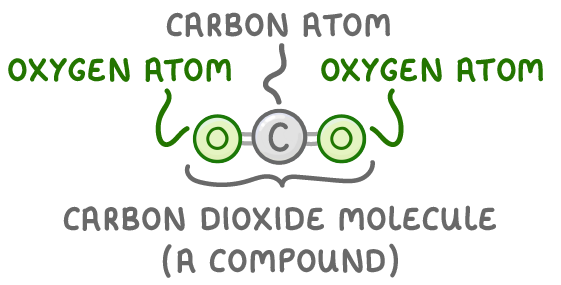
Carbon and oxygen atoms join to form a molecule of carbon dioxide.
The atoms that are chemically joined are from different elements, so it’s a compound.
How do chemical reactions form compounds?
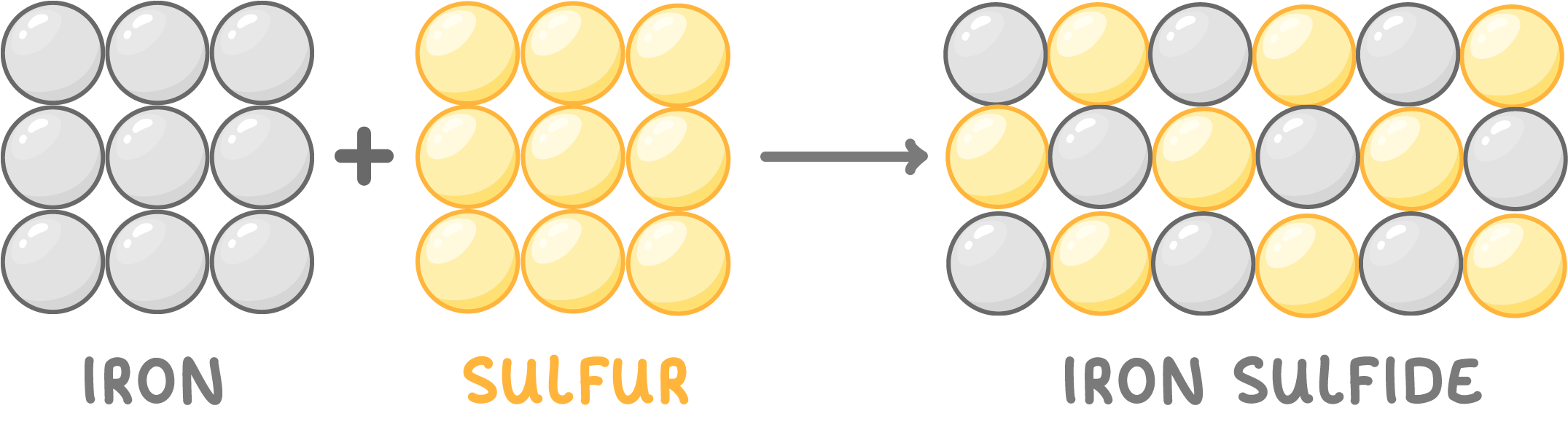
Compounds are formed through chemical reactions when reactants combine together or split apart to make new substances called products.
In a reaction, elements undergo rearrangement of their atoms to produce compounds with new properties.
For example, the reaction between iron and sulfur forms the compound iron sulfide.
Properties of compounds

Compounds have different physical and chemical properties from the original elements they were formed from.
- Iron is a strong, shiny, and malleable metal that is magnetic.
- Sulfur is a brittle, yellow, non-metallic solid that burns easily.
- However, iron sulfide is a black brittle solid that is not magnetic.