Separating mixtures
This lesson covers:
- Filtration and evaporation
- Chromatography
- Distillation
- Using melting and boiling points to check purity
Filtration and evaporation
The sand and the salt in the mixture rock salt have different solubilities
If we've got a mixture of sand and salt, we can use filtration and evaporation to separate them.
Separating rock salt - step 1
- First grind up the rock salt into smaller grains. This increases the surface area.
Separating rock salt - step 2
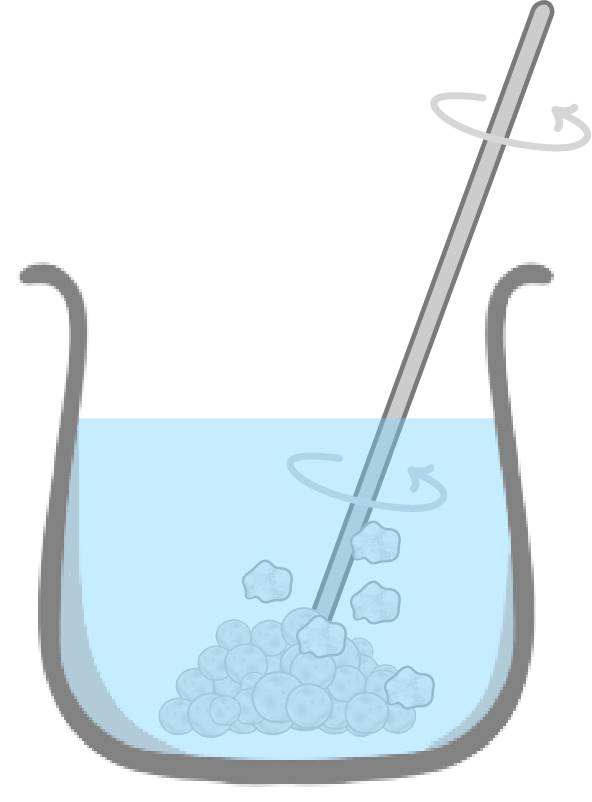
- Next, add the rock salt to water and stir. The salt is soluble and dissolves readily in water, but sand is insoluble and does not.
Separating rock salt - step 3

- Then pour the salt solution through a filter, like filter paper. This removes large insoluble sand particles, leaving just the dissolved salt in the water.
Separating rock salt - step 4
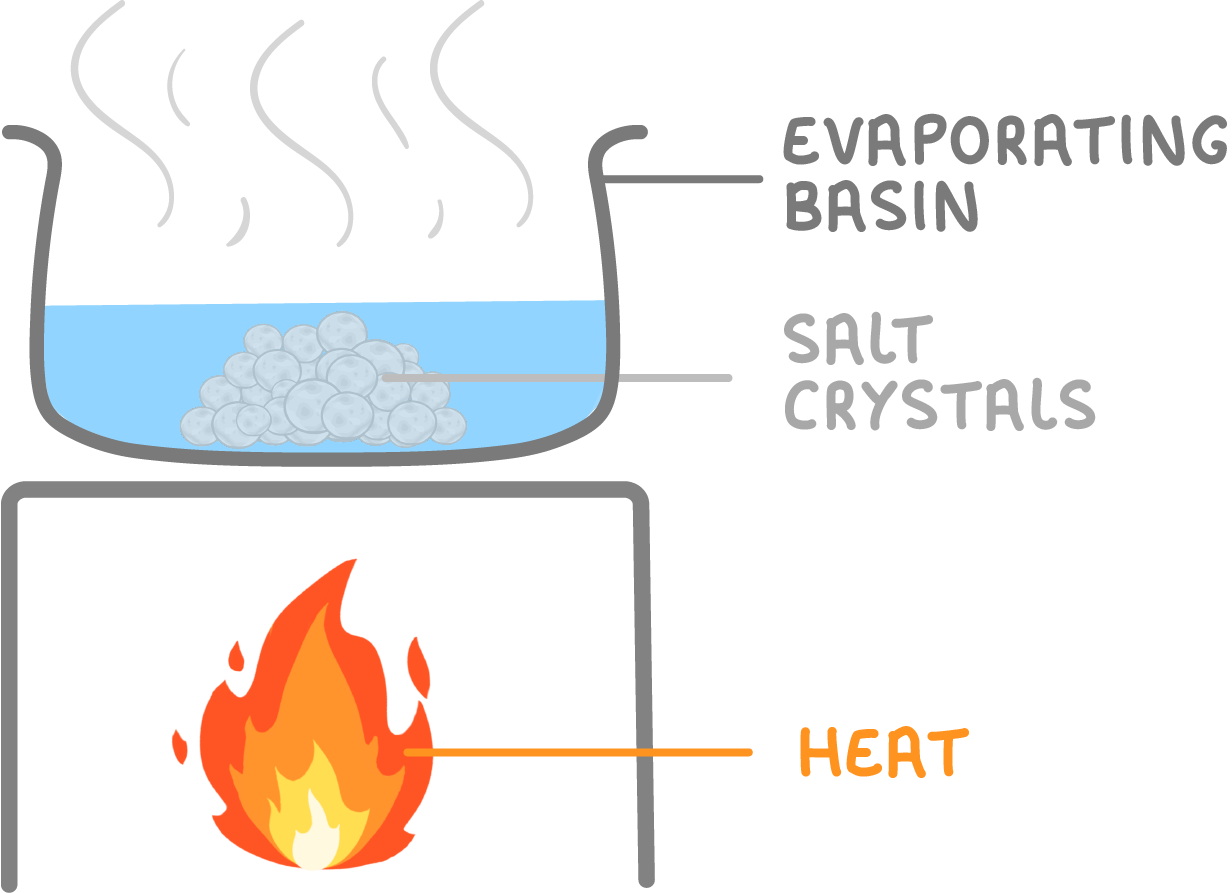
- Finally, gently heat the solution until all the water evaporates away. This leaves behind pure salt crystals, completely separated from the sand.
Chromatography
Chromatography is a great technique for separating out the different dyes in an ink mixture.
It works because of the dyes' different solubilities.

- Put ink spots on paper and place the bottom of the paper in the solvent.
- The solvent moves up the chromatography paper.
- Dyes that are more soluble in the solvent move further up the paper.
- This results in visible separation into bands at different distances up the paper.
Distillation
Distillation uses differences in boiling points to separate liquid mixtures.
Simple distillation
Simple distillation is used to separate a water from a solution.
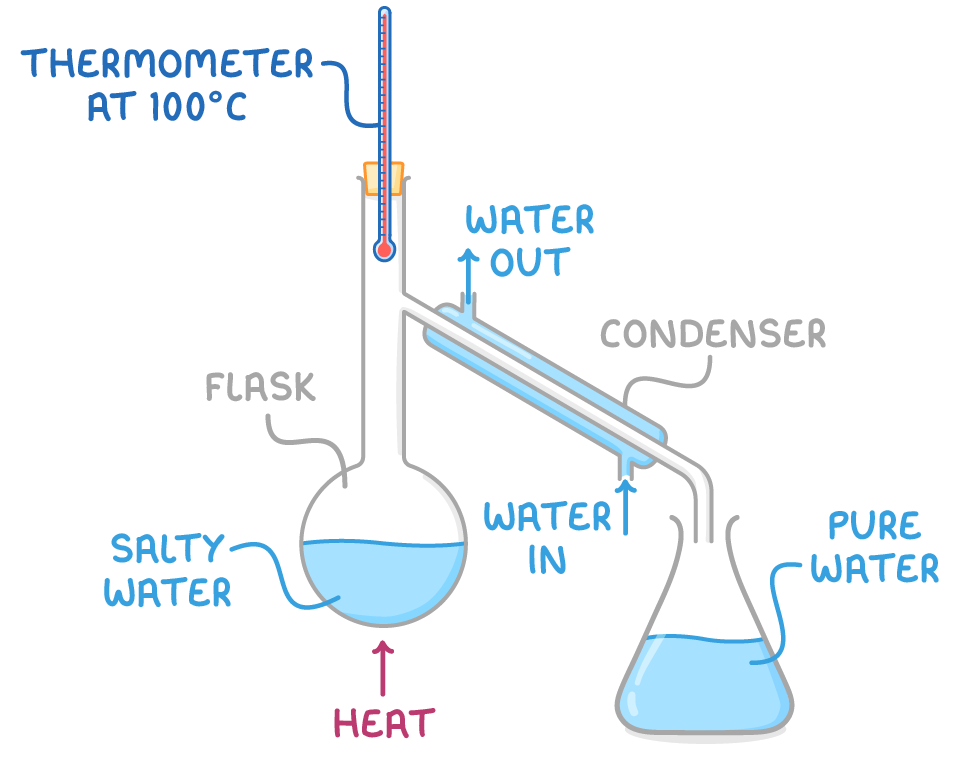
- The impure liquid mixture is heated in a flask until it boils.
- The water vapour rises, enters the condenser and cools, condensing back into a liquid.
- Pure water drips out of the condenser and can be collected in a separate container.
- Impurities are left behind in the original flask as they require a higher temperature to boil.
Fractional distillation
Fractional distillation is used to separate mixtures of liquids with different boiling points.
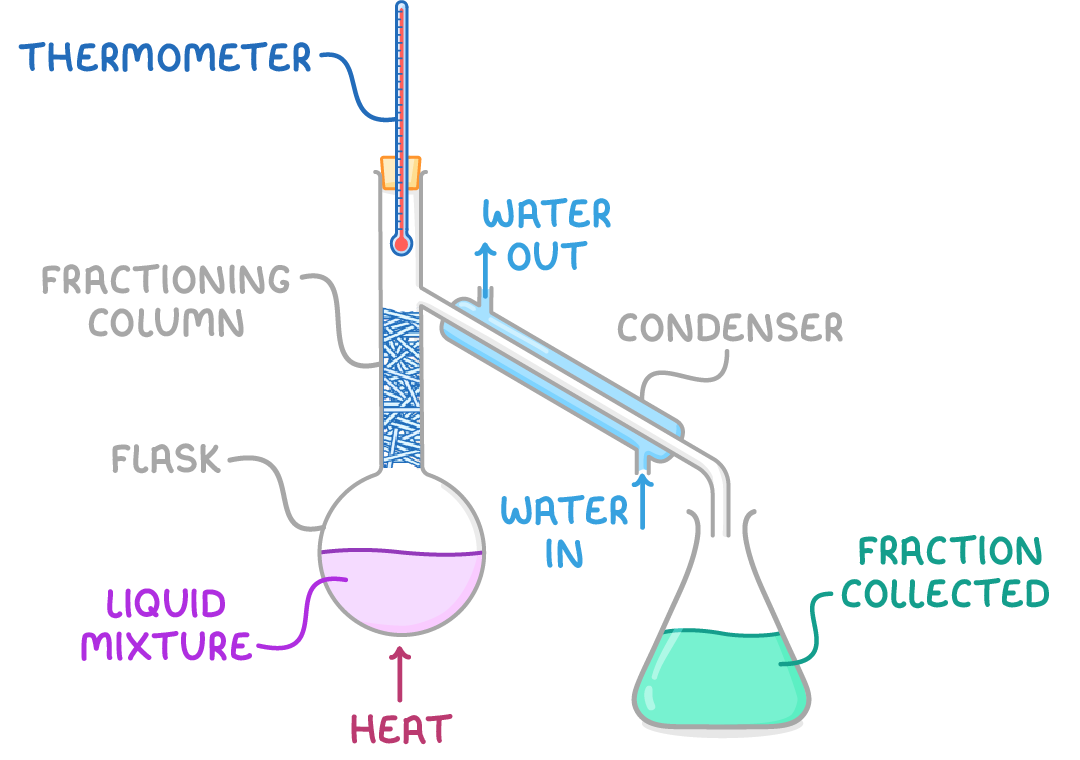
- The liquid mixture is heated in a flask.
- As the temperature rises, the fraction with the lowest boiling point boils first.
- When the gas reaches the condenser it is cooled and returns to a liquid.
- The condensed liquid is collected in a separate container.
- As the temperature rises further, the next fraction starts to boil and condense.
- This process results in separation of the mixture into fractions based on boiling point.
Melting and boiling points
Pure substances have defined melting and boiling points that do not change.
For example: pure water melts at 0°C and boils at 100°C
If a substance contains impurities, the melting and boiling points will be different from the pure substance.
Scientists can test the melting and boiling points of a separated substance to check it’s purity.