Properties of non-metals
This lesson covers:
- Location of non-metals on the periodic table
- Properties of non-metals
Non-metals in the periodic table
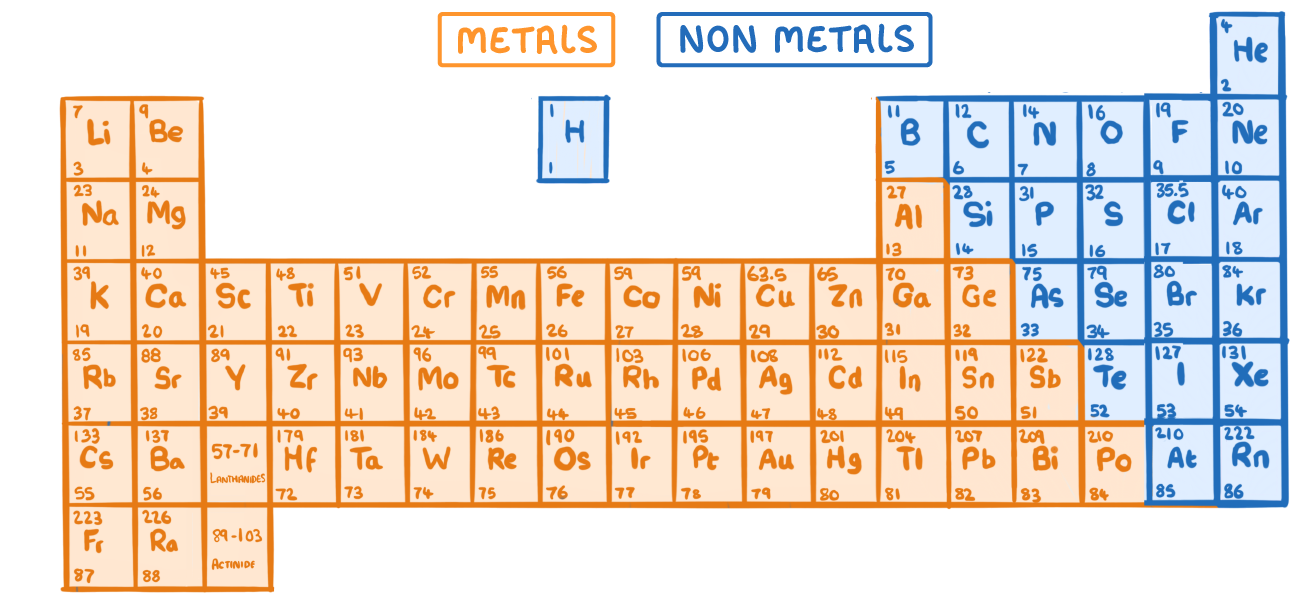
Only some of the elements found in the periodic table are non-metals.
Non-metals are found on the right side of the periodic table.
Properties of non-metals
Non-metals have distinct properties that distinguish them from metals.
Non-metals are electrical insulators
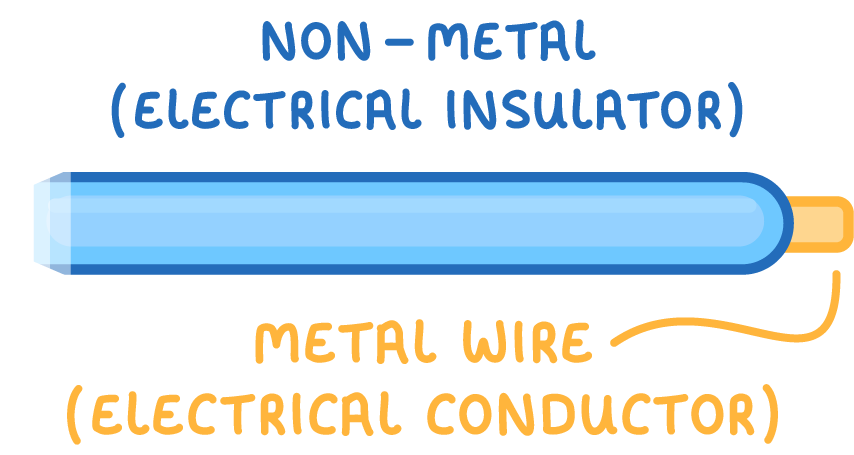
- Non-metals have electrons that are fixed in place and cannot move freely.
- This makes non-metals unable to conduct electricity.
- This means non-metals are useful as insulators in electrical appliances.
Non-metals are thermal insulators
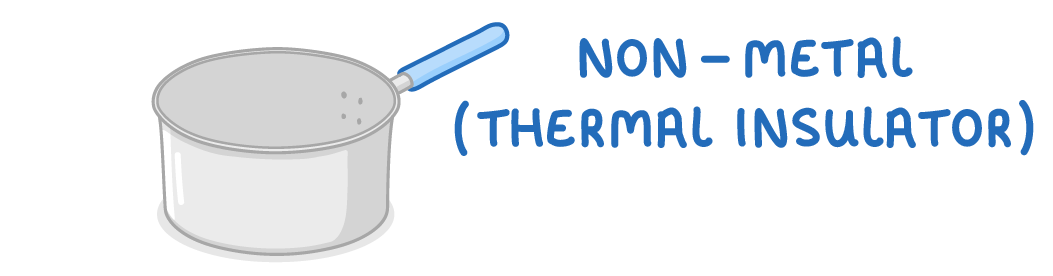
- Non-metals have weak intermolecular forces so cannot readily conduct heat energy.
- This makes them useful as insulators for heat.
- This means non-metals do not easily transfer heat from hot to cold areas.
Non-metals are brittle and weak

- Non-metals have weak intermolecular bonds so they fracture and shatter easily.
- This makes them brittle materials that break under stress.
Non-metals are dull

- Non-metals have irregular, surfaces that do not reflect light well.
- This results in non-metals having a dull, non-reflective appearance.
Non-metals have low melting and boiling points

- Weak bonds between particles mean non-metals melt and boil at low temperatures.
- Most are gases or solids at room temperature.
Non-metals have low density
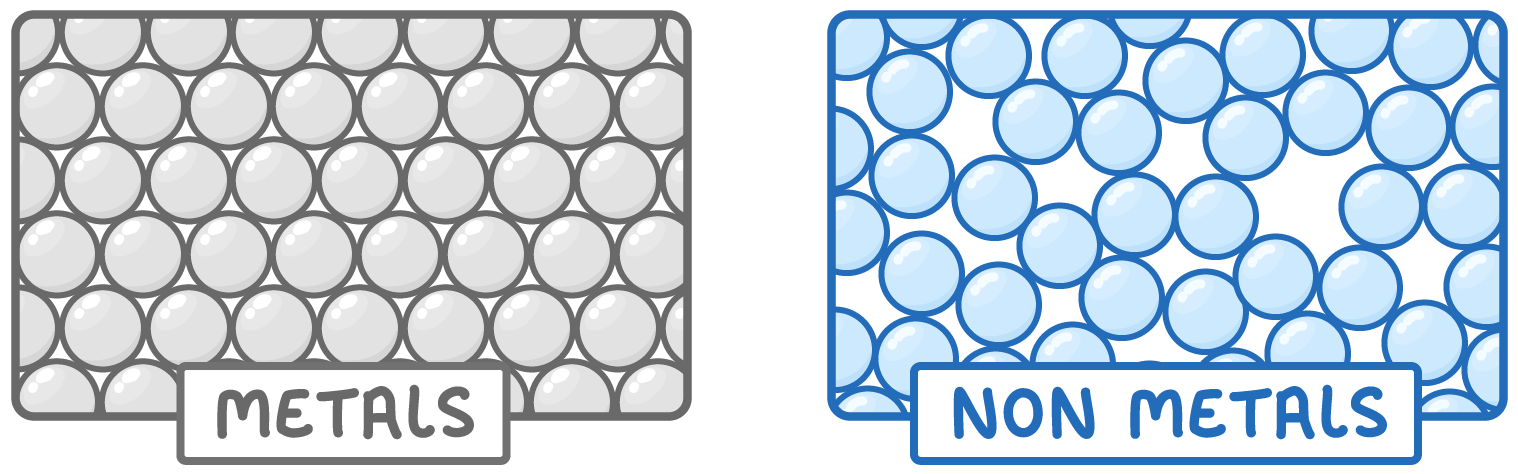
The atoms in non-metals are typically less tightly packed than those of metals. This means they are less dense, and usually weigh less than the same volume of metal.
- Gaseous non-metals have very low densities and will float.
- Even solid non-metals have lower densities than metals.
Non-metals are not magnetic
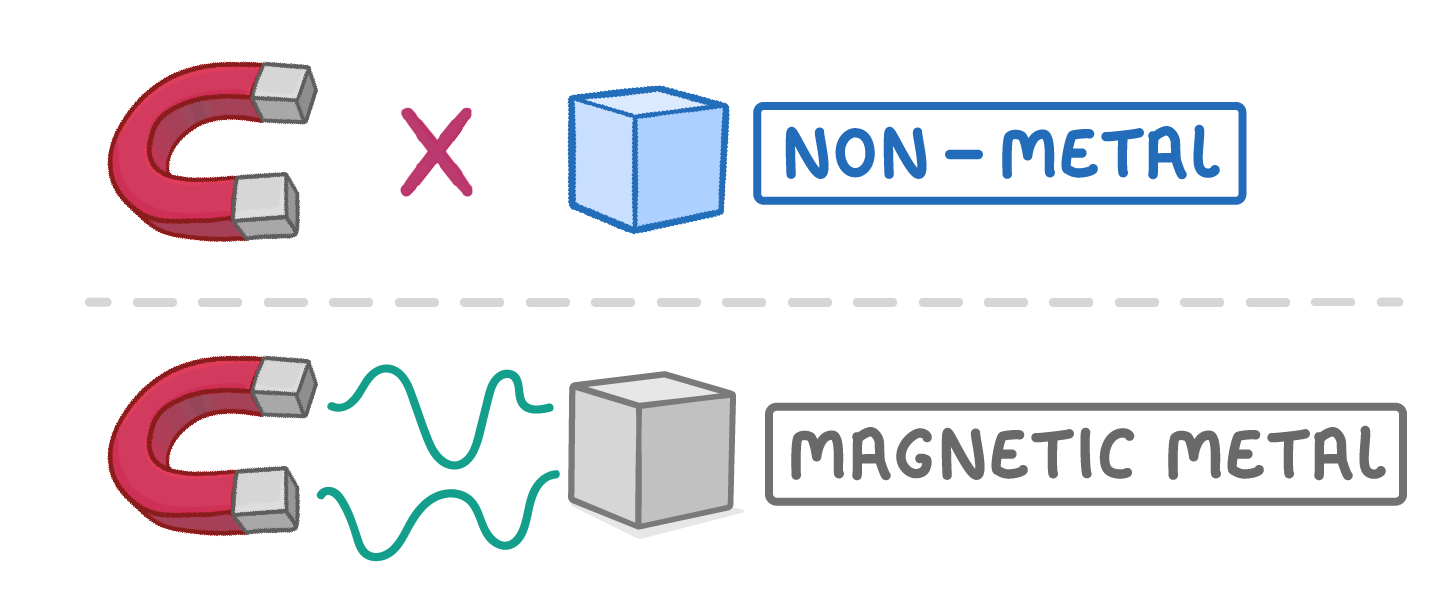
- Non-metals are not magnetic, unlike some metals.
- This distinguishes them from magnetic metals like iron.