Solids, liquids and gases
This lesson covers:
- The three states of matter
- Properties of solids, liquids and gases
The three states of matter
Matter exists in three main states - solid, liquid and gas.
The state depends on:
- Temperature
- Pressure
- How strongly the particles are bonded
So heating or cooling a material, or changing the pressure, can cause it to change state.
Properties of solid, liquids and gases
We can tell solids, liquids and gases apart by testing their properties.
Properties of solids
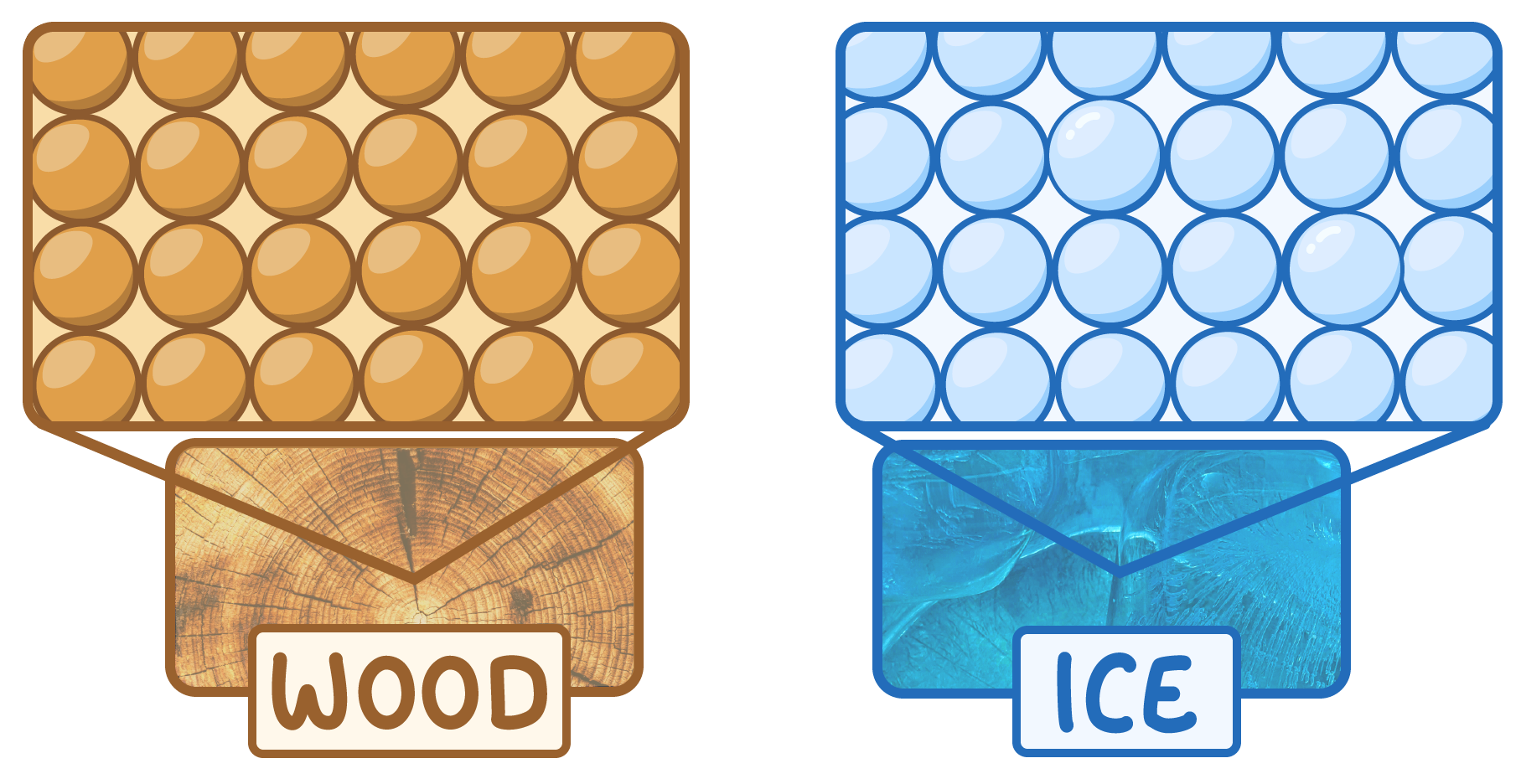
Solids have tightly packed particles. Examples include: wood, ice, and rocks.
This means that solids have the following properties:
- A definite volume and shape
- Very high density
- Cannot be compressed
- Do not flow
Properties of liquids
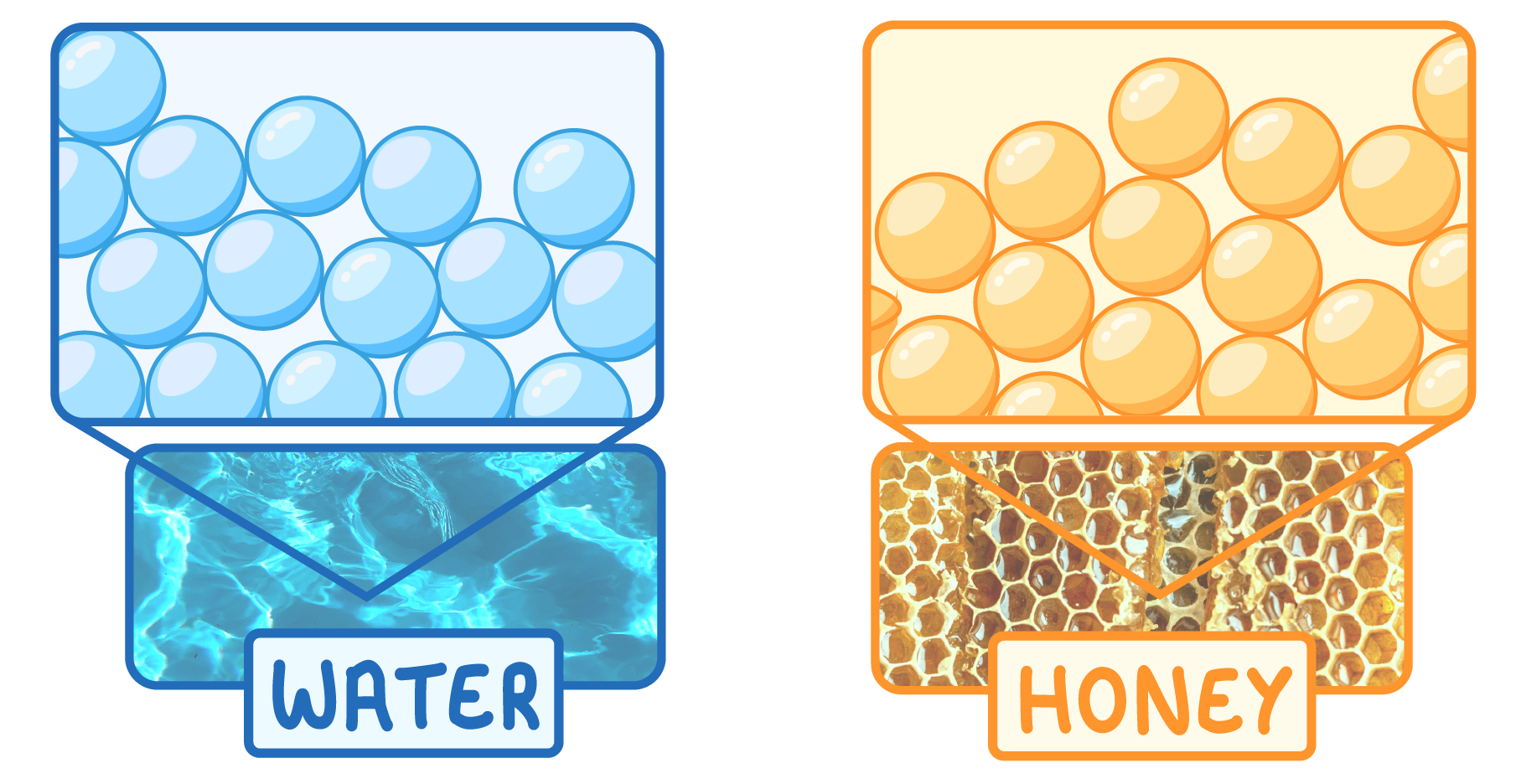
Liquids have particles that can slide past each other. Examples include: water, honey, and oil.
This means that liquids have the following properties:
- Take the shape of the container
- Medium density
- Can’t be compressed
- Can flow easily
Properties of gases
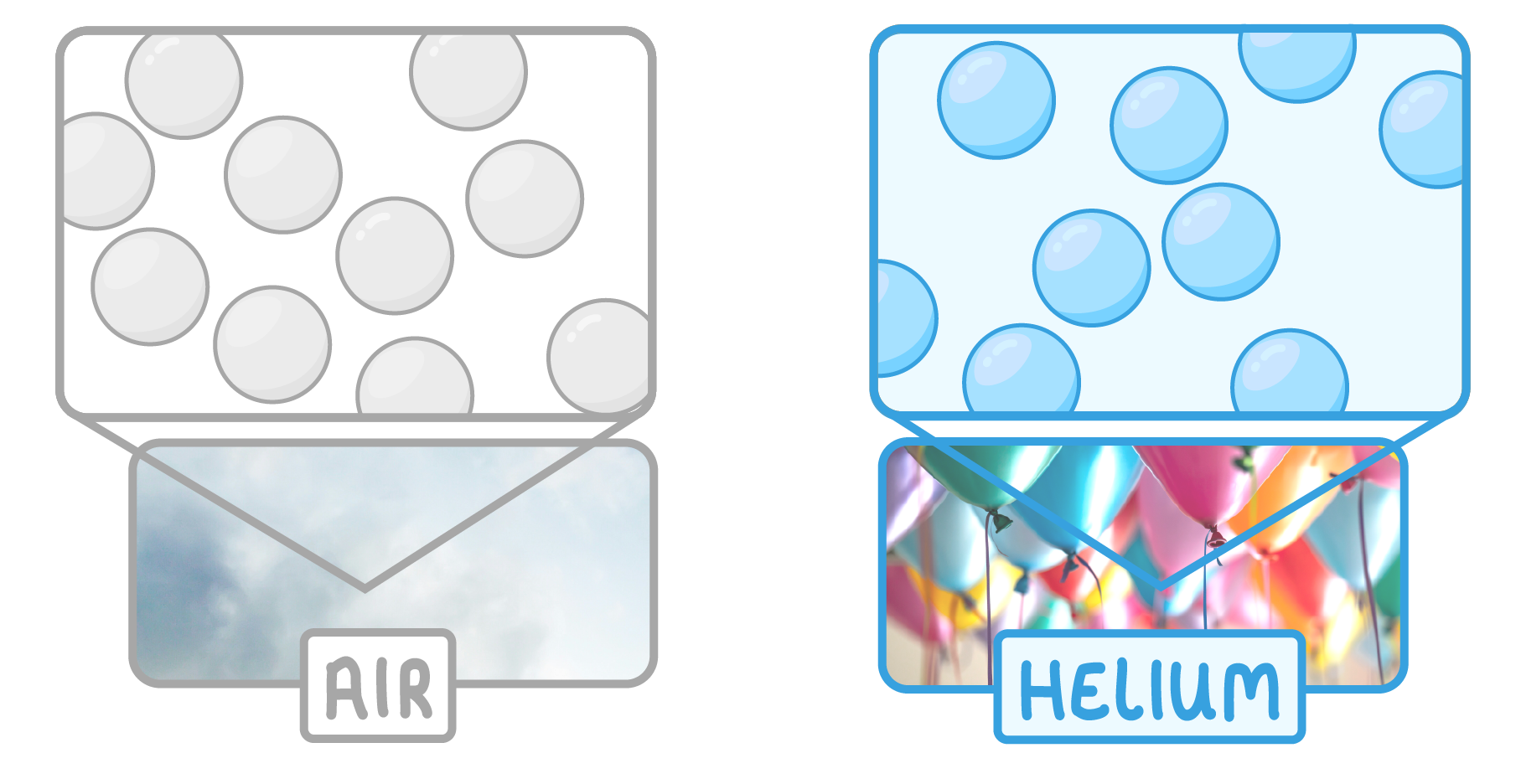
Gases have freely moving particles. Examples include: air, helium, and steam (which is the gas form of water).
This means that gases have the following properties:
- Can fill any container
- Very low density
- Easily compressed
- Can flow and mix with ease