Naming compounds
This lesson covers:
- Naming compounds made from two elements
- Naming compounds made from more than two elements
- Elements that exist as molecules
Naming compounds made from two elements
When two different elements join to form a compound, the naming rules are pretty simple:
- The first element keeps its name the same.
- The second element gets the suffix -ide added to the end.
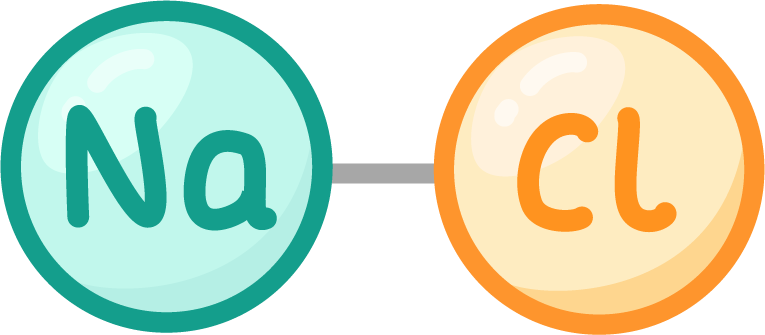
For example sodium chloride:
- Formula - NaCl.
- Elements - 1 sodium atom, 1 chlorine atom.
- Name of compound - Sodium chloride.
Some other common examples are:
- Sulfur becomes sulfide.
- Iodine becomes iodide.
- Bromine becomes bromide.
- Fluorine becomes fluoride.
Naming compounds made from more than two elements
When three elements join to form a compound and one of them is oxygen, we name the compound by:
- Keeping the first element name the same.
- Adding the suffix -ate to the oxygen.
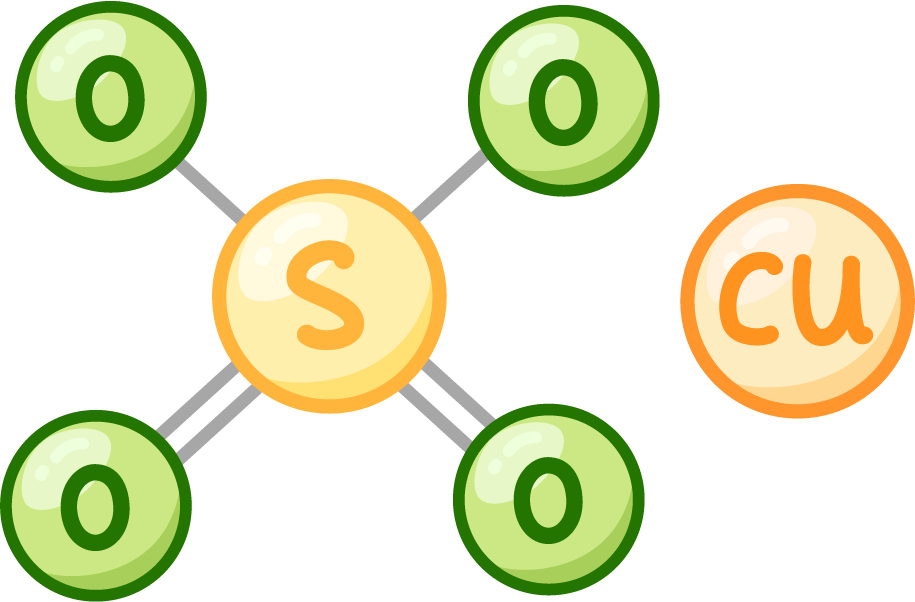
For example copper sulfate:
- Formula - CuSO4.
- Elements - 1 copper atom, 1 sulfur atom, 4 oxygen atoms.
- Name of compound - Copper sulfate.
Some other examples are:
- Sodium + Carbon + 3 Oxygen atoms becomes sodium carbonate.
- Potassium + Sulfur + 4 Oxygen atoms becomes potassium sulfate.
- Ammonium + Nitrogen + 3 Oxygen atoms becomes ammonium nitrate.
Some elements can exist as molecules
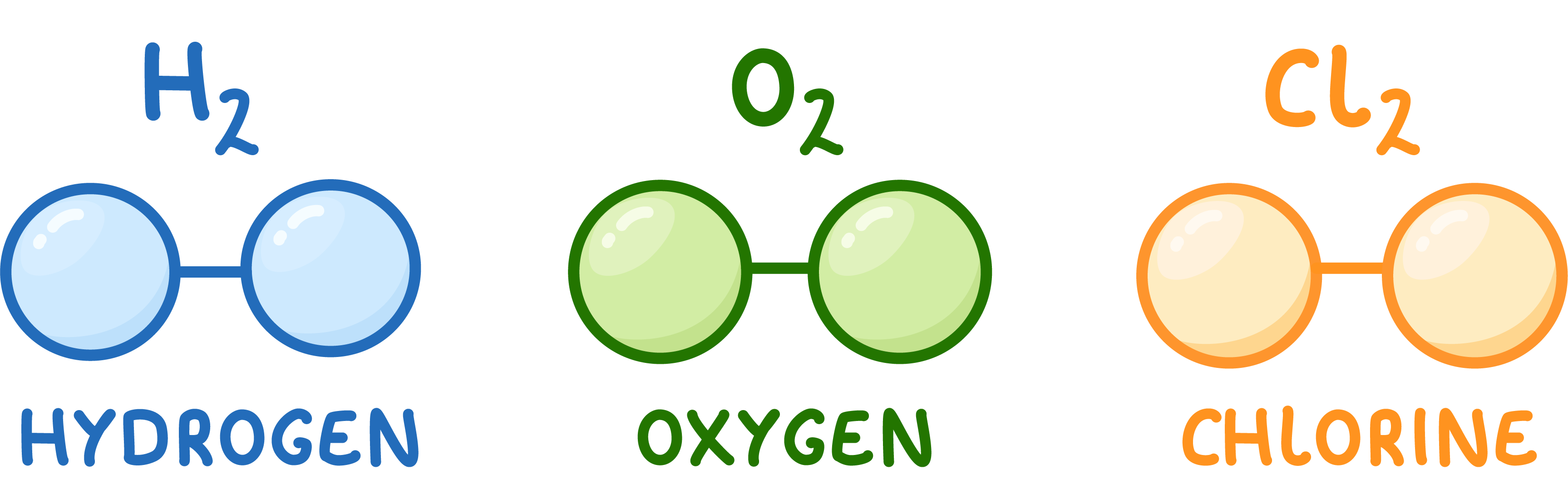
Some elements like hydrogen, oxygen, and chlorine naturally exist as pairs of atoms.
These are still elements, not compounds. So their names stay the same.