The reactivity series
This lesson covers:
- The reactivity series of metals
- Methods for extracting metals
The Reactivity Series
The reactivity series orders metals by how readily they react with other substances.
Carbon and hydrogen are often also included to provide a comparison.
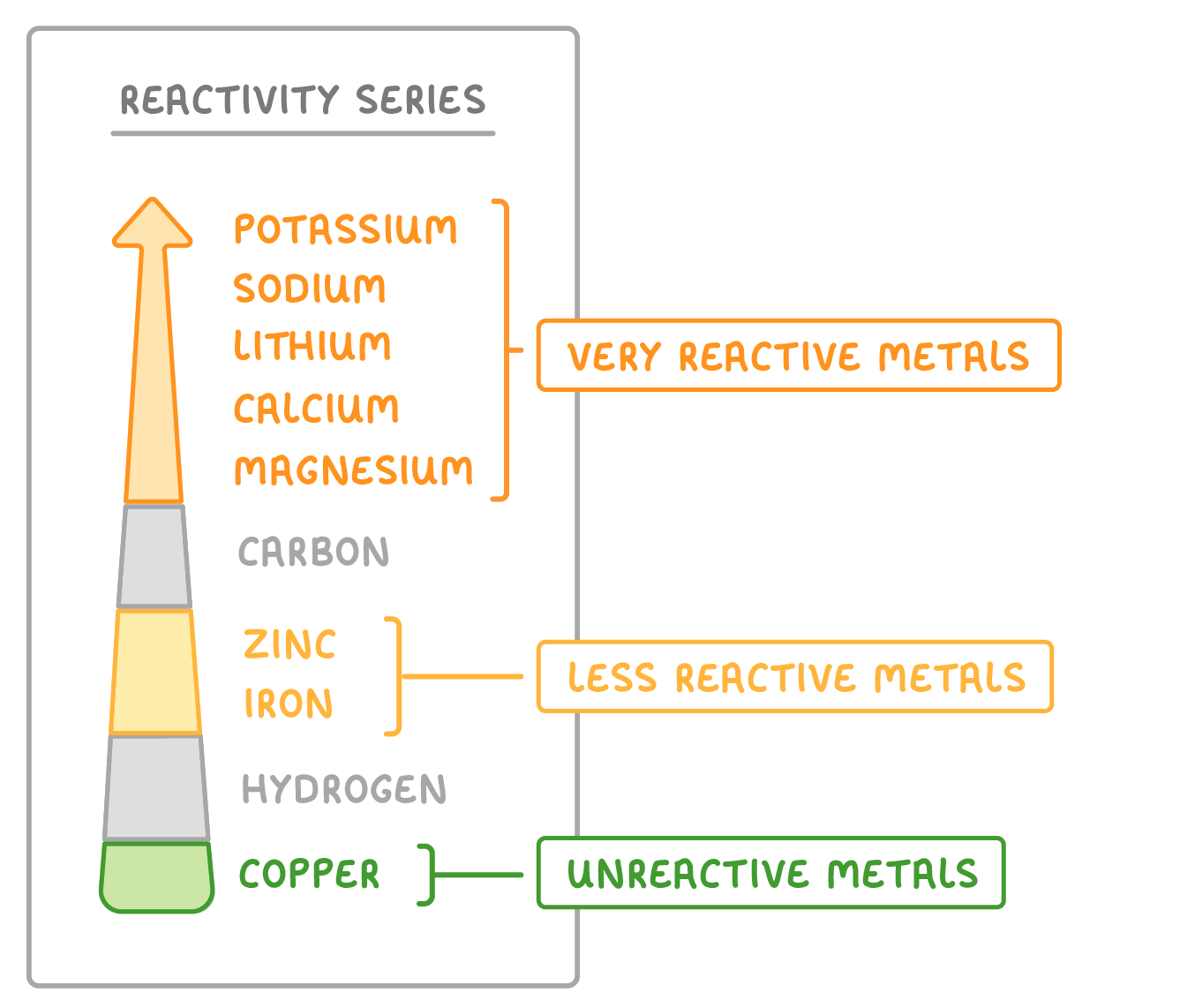
- Metals at the top of the series such as potassium, sodium, and calcium are very reactive.
- Metals at the bottom of the reactivity series such as copper, silver ,and gold are not reactive.
Methods for Metal Extraction
The extraction method depends on reactivity of the metals.
Some metals are extracted by electrolysis
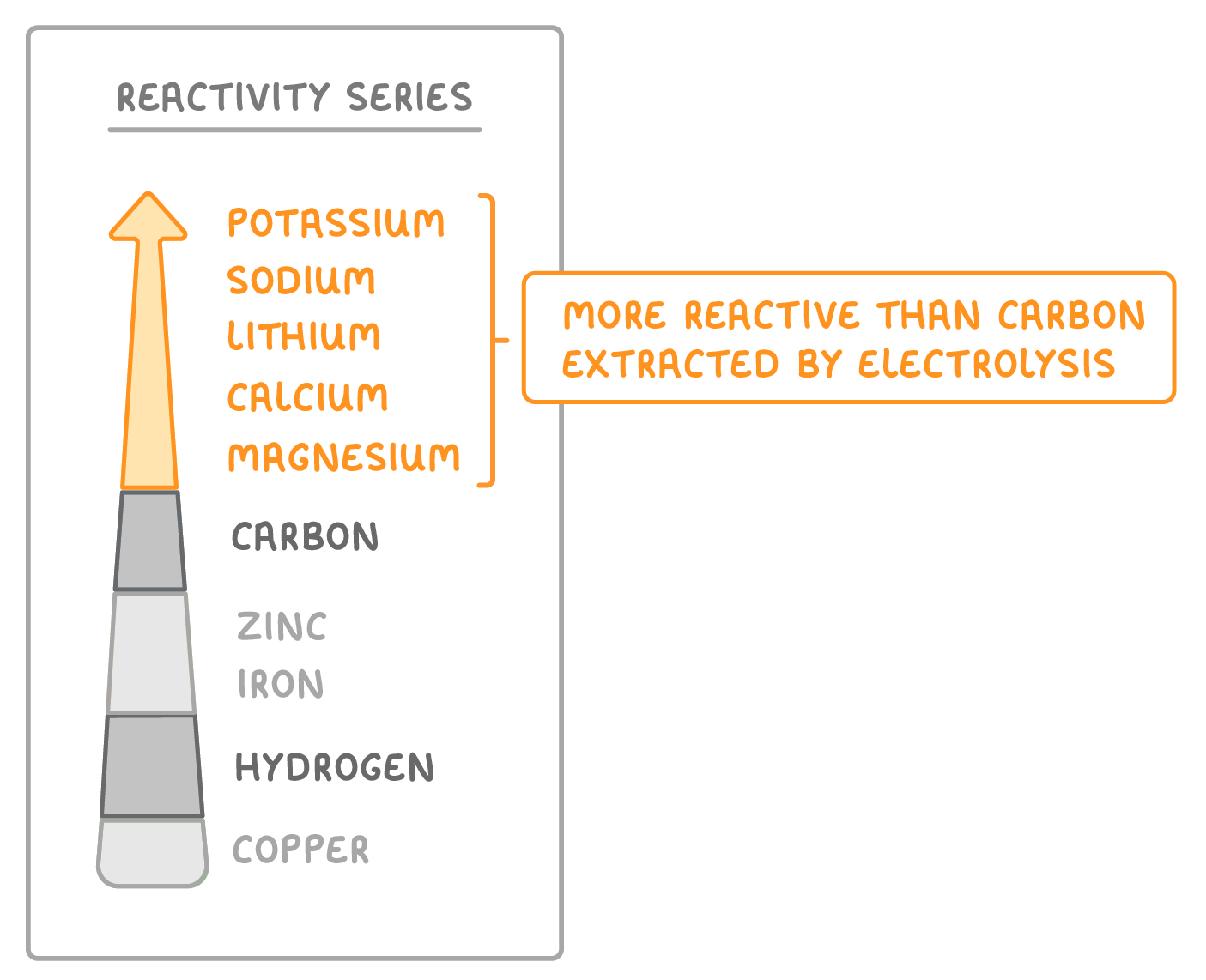
- Metals more reactive than carbon are extracted by electrolysis.
- Electricity splits the ore compounds to extract the metal.
- Potassium, sodium, calcium, magnesium, and aluminium are extracted by electrolysis.
Some metals are extracted by reduction with carbon
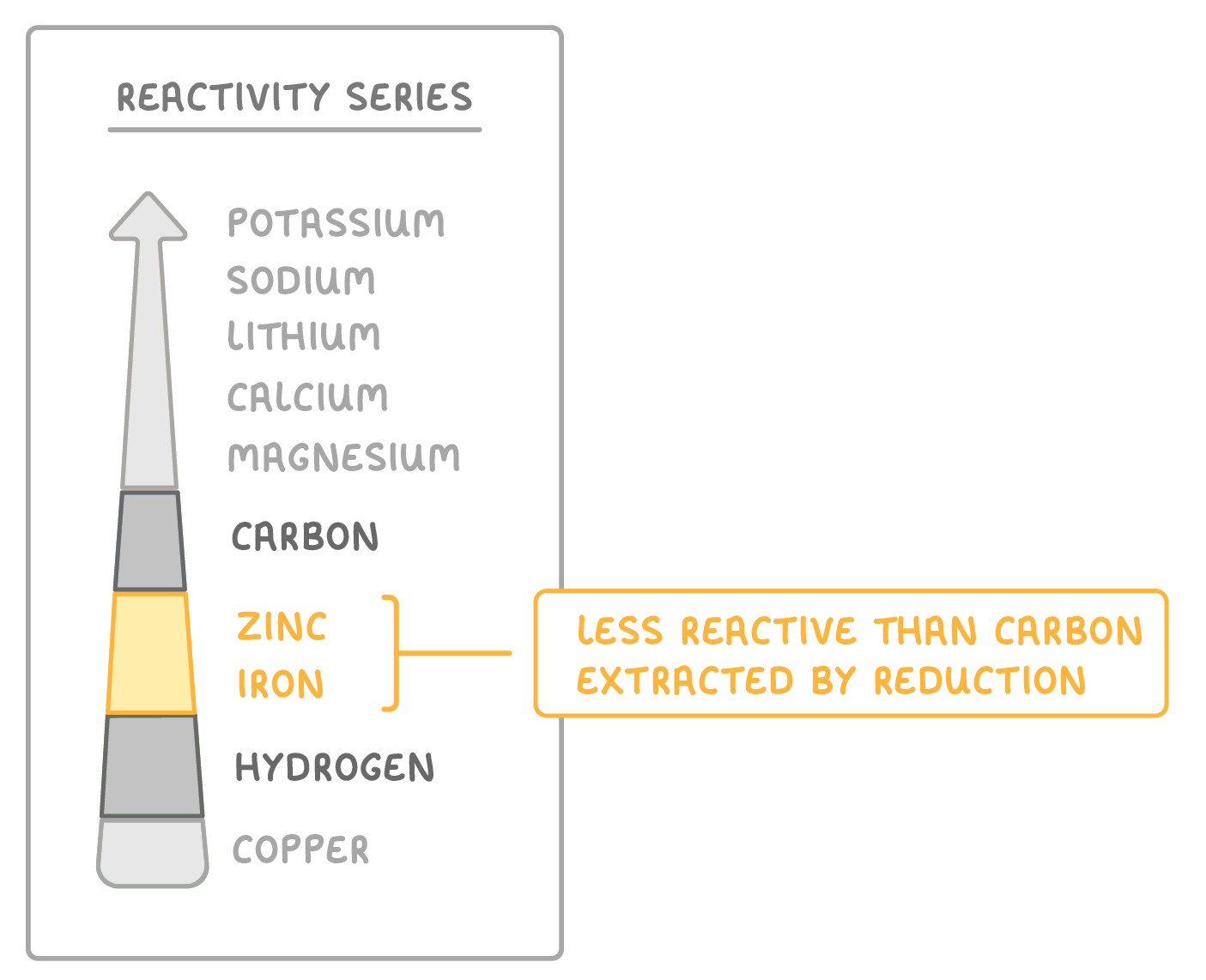
- Metals less reactive than carbon and more reactive than hydrogen are extracted using reduction with carbon.
- Heating the metal ore with carbon removes oxygen as CO2, leaving pure metal behind.
- Metals zinc, iron, tin, and lead are extracted by reduction with carbon.
Some metals are found in their native state
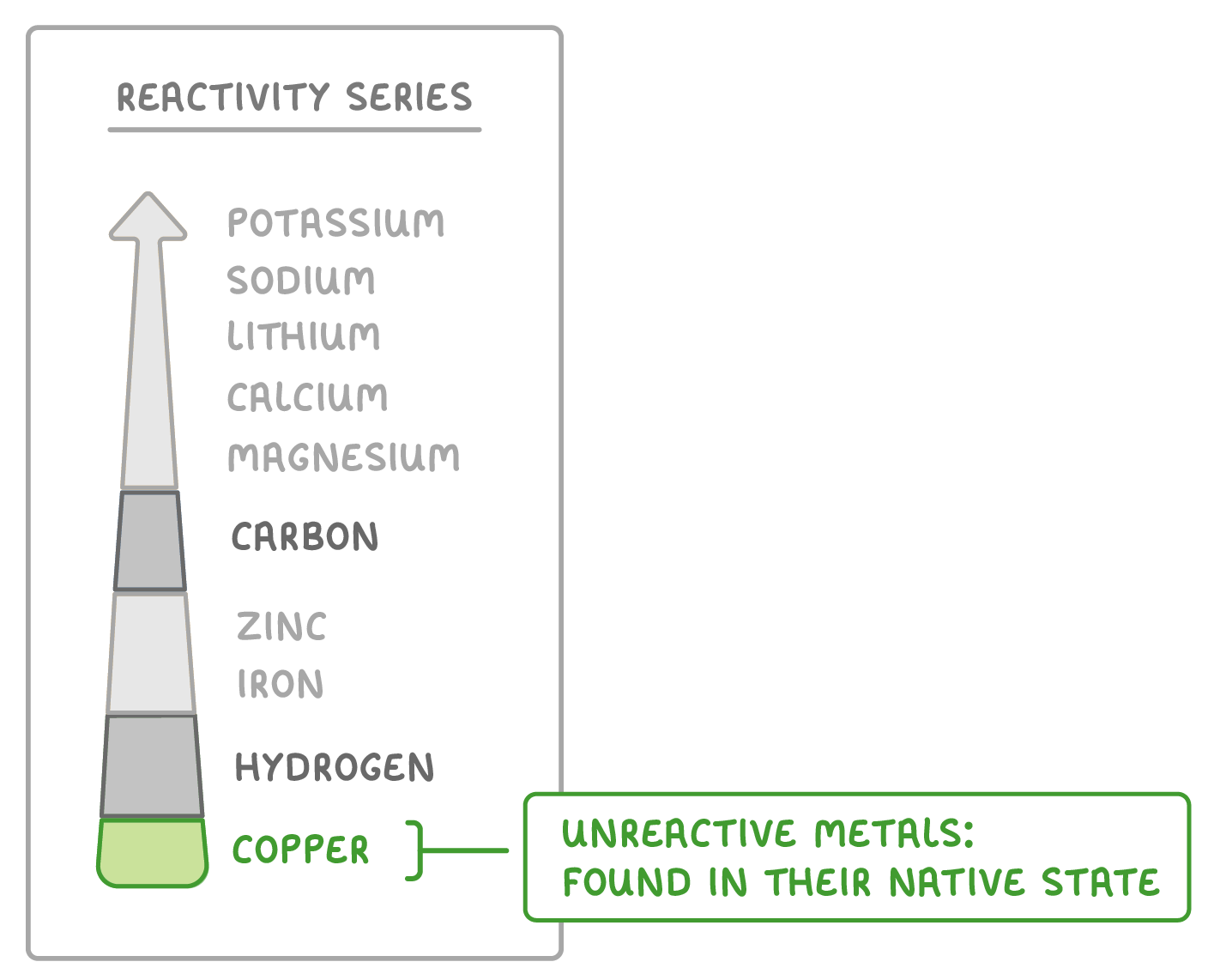
- Metals less reactive than hydrogen are found in their native state.
- No extraction is required.
- Metals copper, silver, gold, and platinum are found as pure elements.