Displacement reactions
This lesson covers:
- Predicting displacement reactions
- Neutralisation as a displacement reaction
- A reactivity series investigation
Predicting Displacement Reactions
The reactivity series lists metals from most reactive to least reactive.
You can use it to predict whether one metal will displace another from its compound in a displacement reaction.
- Displacement means one metal takes the place of another in a compound.
- A more reactive metal will displace a less reactive metal from its compound.
Magnesium displaces copper
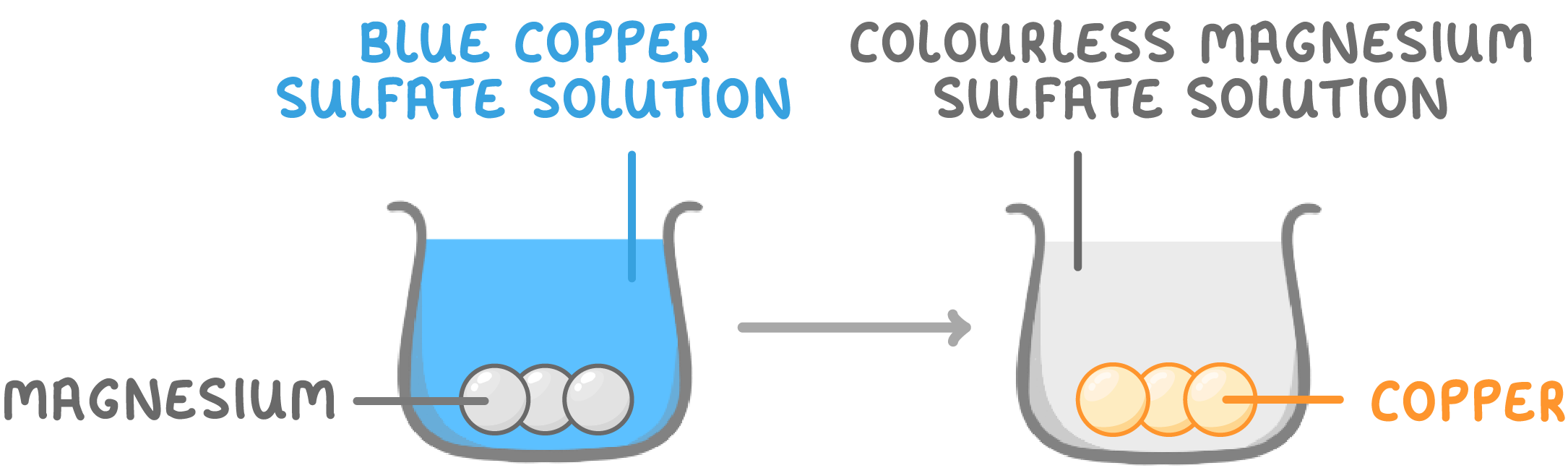
For example:
- Magnesium is more reactive than copper (higher on the reactivity series).
- If magnesium is added to a copper sulfate solution:
- Magnesium displaces copper from the copper sulfate.
- Magnesium takes copper's place, making magnesium sulfate.
- The displaced copper coats the magnesium.
This reaction can be represented by the equation:
magnesium + copper sulfate → magnesium sulfate + copper
- The displacement only happens if the added metal is more reactive (higher on the reactivity series).
Neutralisation is a displacement reaction
The reaction between an acid and an alkali is a type of displacement reaction.
For example, in the reaction between hydrochloric acid and sodium hydroxide:
- The hydrogen in the acid is displaced by the sodium in the alkali.
- This makes sodium chloride (NaCl) and water (H2O).
The neutralisation equation is:
hydrochloric acid + sodium hydroxide → sodium chloride + water
Setting Up a Reactivity Series Investigation
An investigation can determine a reactivity series for metals. You can do this by:
- Putting metals into solutions of different metal compounds.
- Observing if displacement reactions occur.
- Arranging metals from most to least reactive based on the observations.
Sample investigation results - tube 1
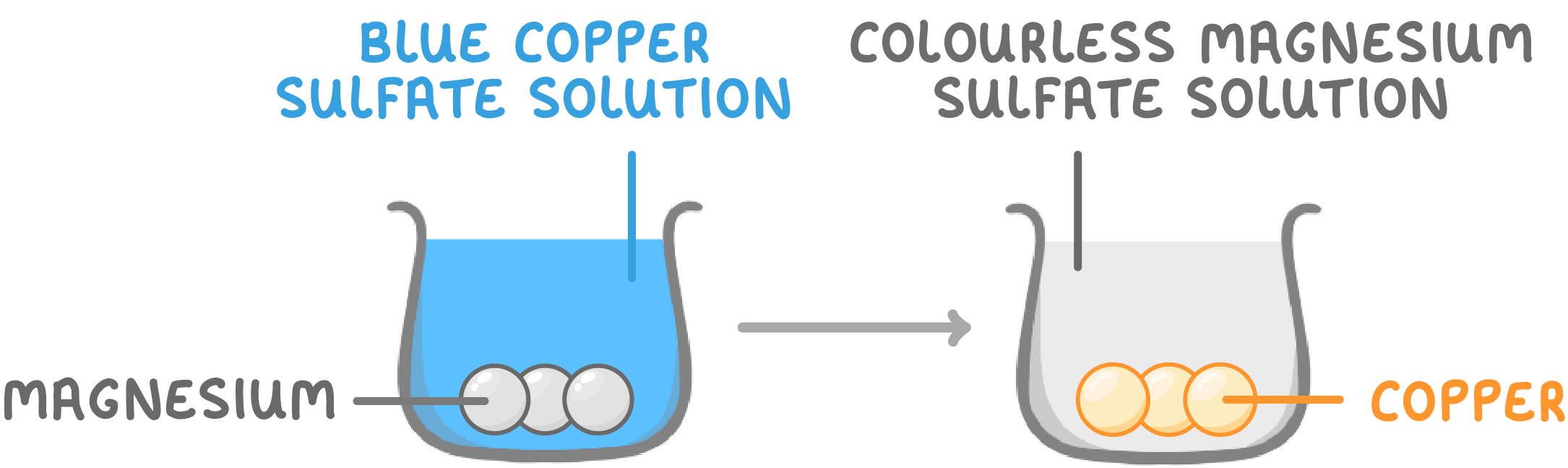
When magnesium is placed in copper sulfate solution:
- Blue copper sulfate solution goes colourless.
- Copper coats the magnesium.
- This is because magnesium displaces copper from the copper sulfate.
- So magnesium is more reactive than copper.
Sample investigation results - tube 2
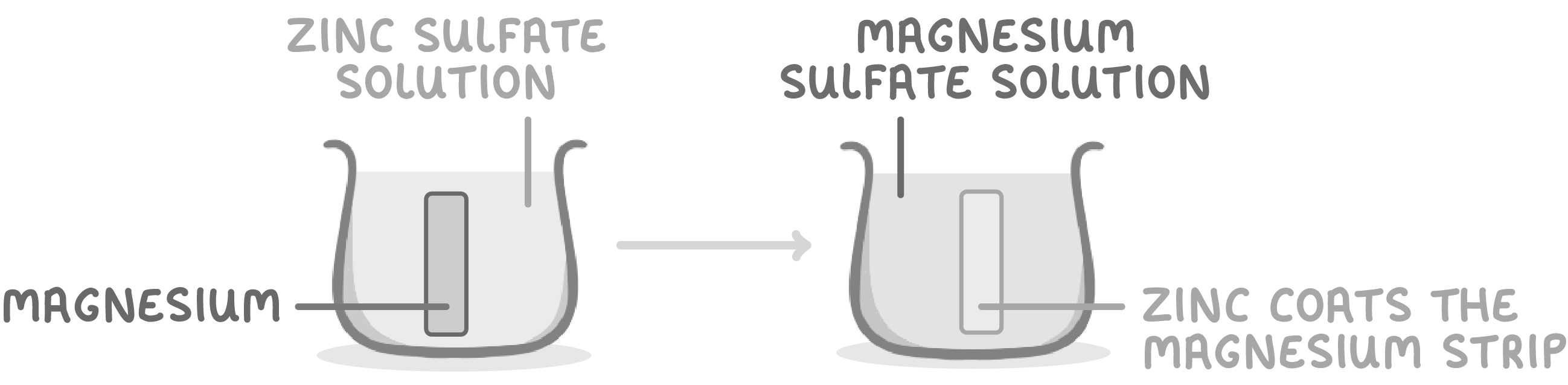
When magnesium is placed in zinc sulfate solution:
- Zinc coats the magnesium strip.
- This is because magnesium displaces zinc from the zinc sulfate.
- So magnesium is more reactive than zinc.
Sample investigation results - tube 3
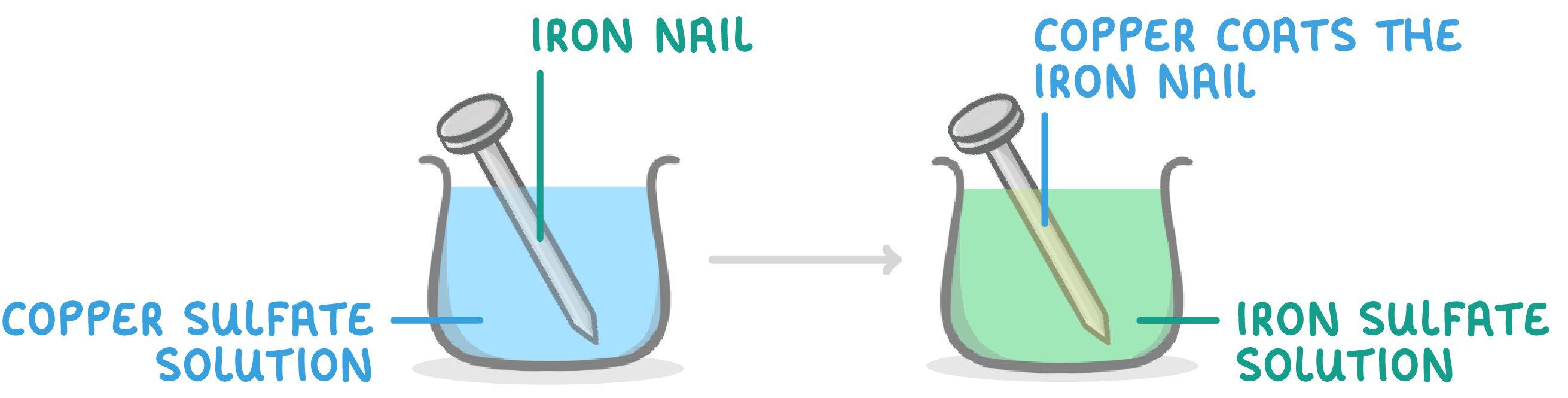
When iron is placed in copper sulfate solution:
- Blue copper sulfate solution goes green.
- Copper coats the iron nail.
- This is because iron displaces copper from the copper sulfate.
- So iron is more reactive than copper.
Sample investigation results - tube 4
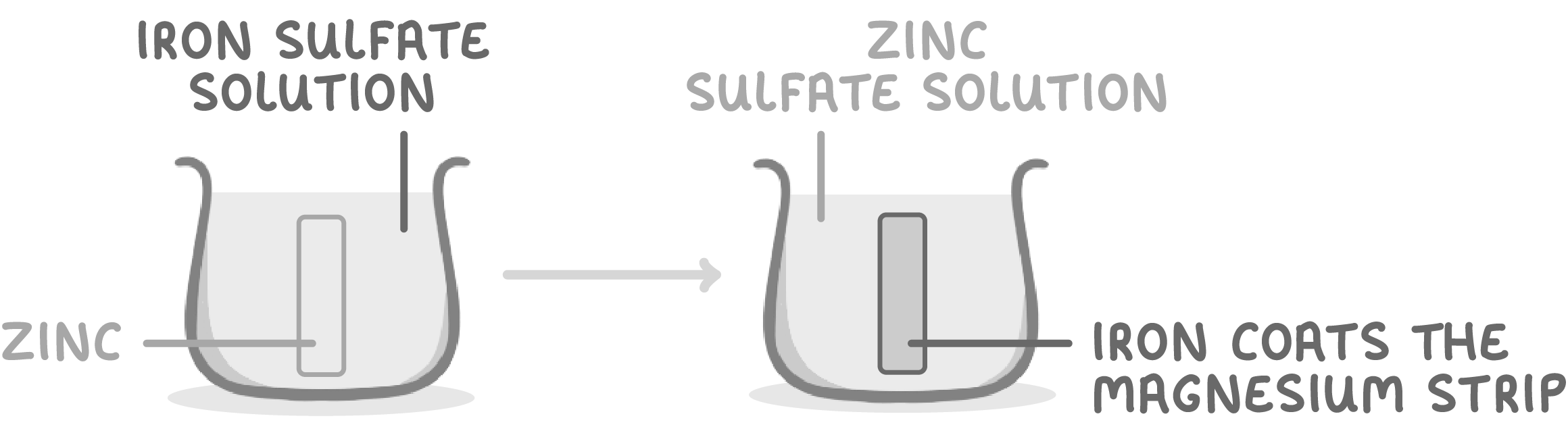
When zinc is placed in iron sulfate solution:
- Iron coats the zinc strip.
- This is because zinc displaces iron from the iron sulfate.
- So zinc is more reactive than iron.
Sample investigation results - tube 5
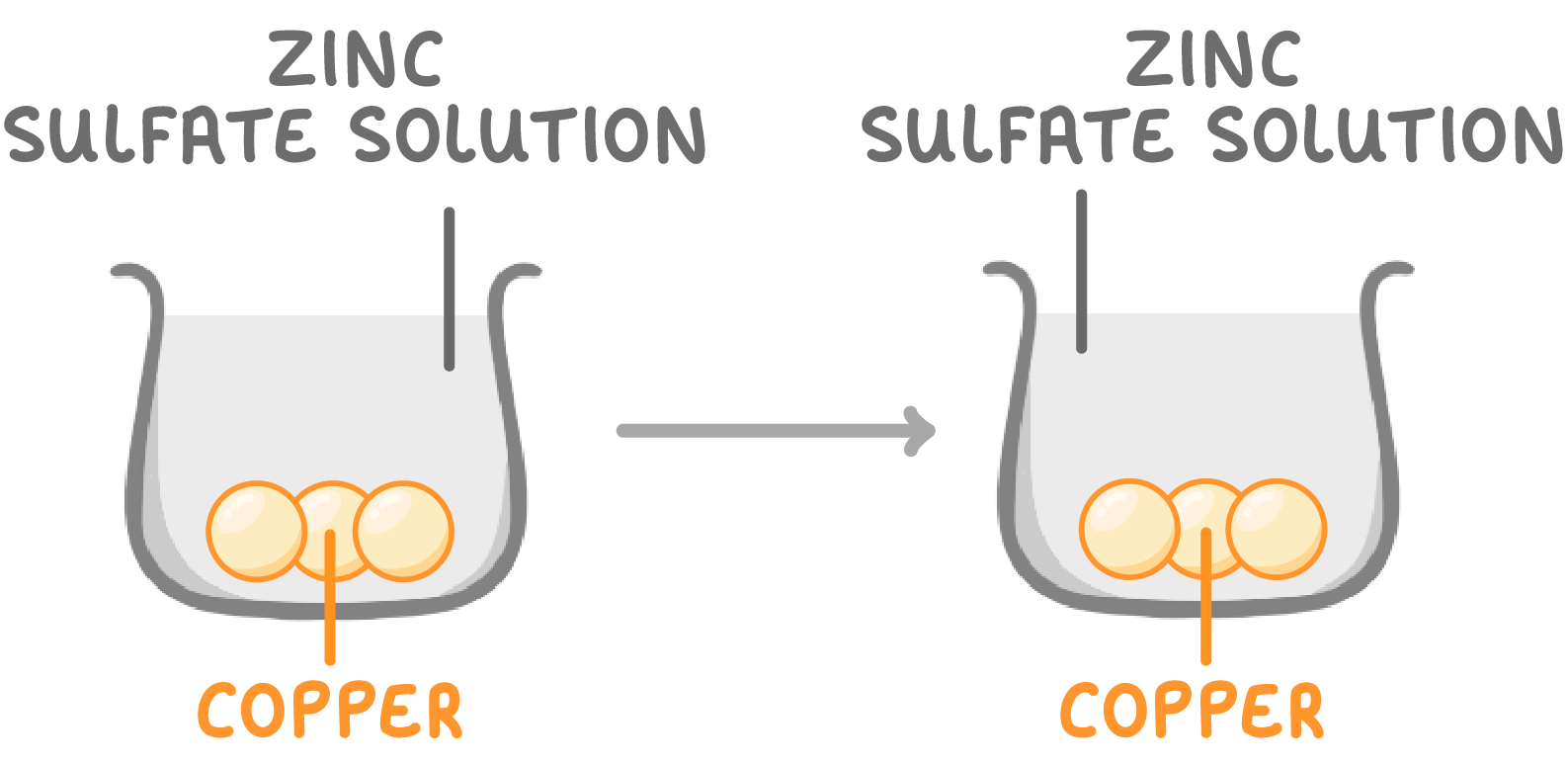
When copper is placed in zinc sulfate solution:
- No reaction occurs between copper and zinc sulfate.
- Copper can't displace zinc - it's not reactive enough.
Conclusion
More reactive metals will always displace less reactive metals from their compounds.
So the outcomes of this experiment can establish the order of reactivity.
Order of Reactivity
- Magnesium (most reactive)
- Zinc
- Iron
- Copper (least reactive)