Physical changes
This lesson covers:
- Differences between physical and chemical changes
- Changes of state as examples of physical changes
- How heating and cooling affects particles during changes of state
Physical changes
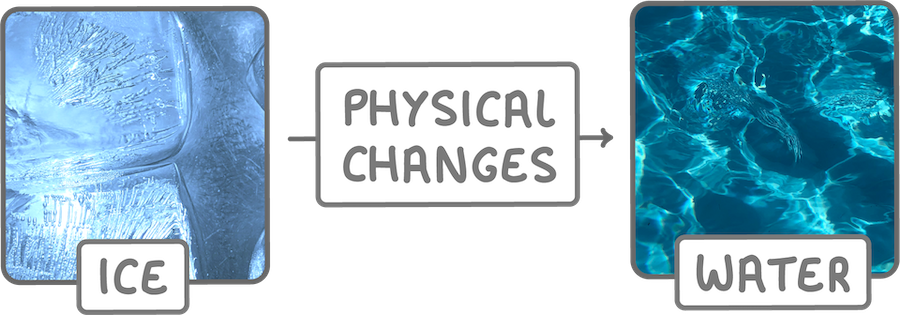
Physical changes rearrange particles or alter their energy levels but do not form new substances.
Examples include changes of state (solid, liquid, gas), dissolving, and breaking objects.
Chemical changes
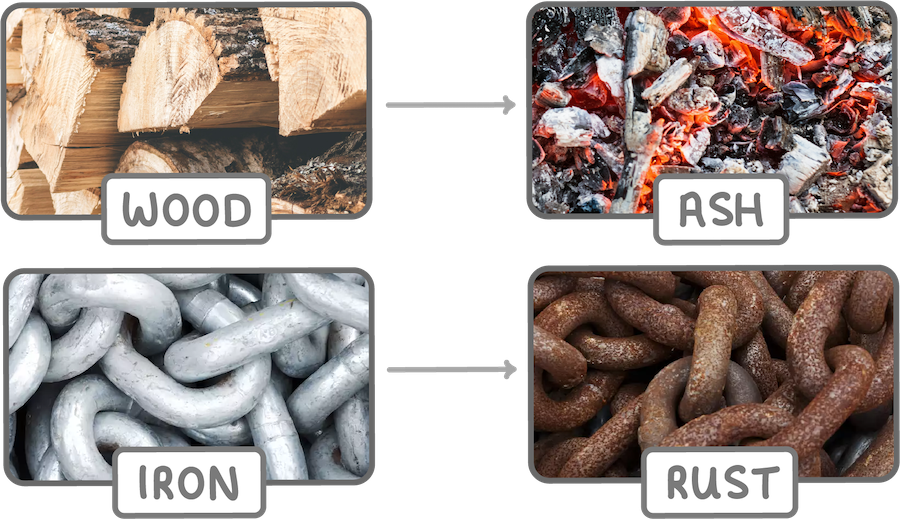
Chemical changes break bonds and form new substances with different properties.
Examples include burning wood into ash, rusting of iron, and food digesting.
Changes of state are physical changes
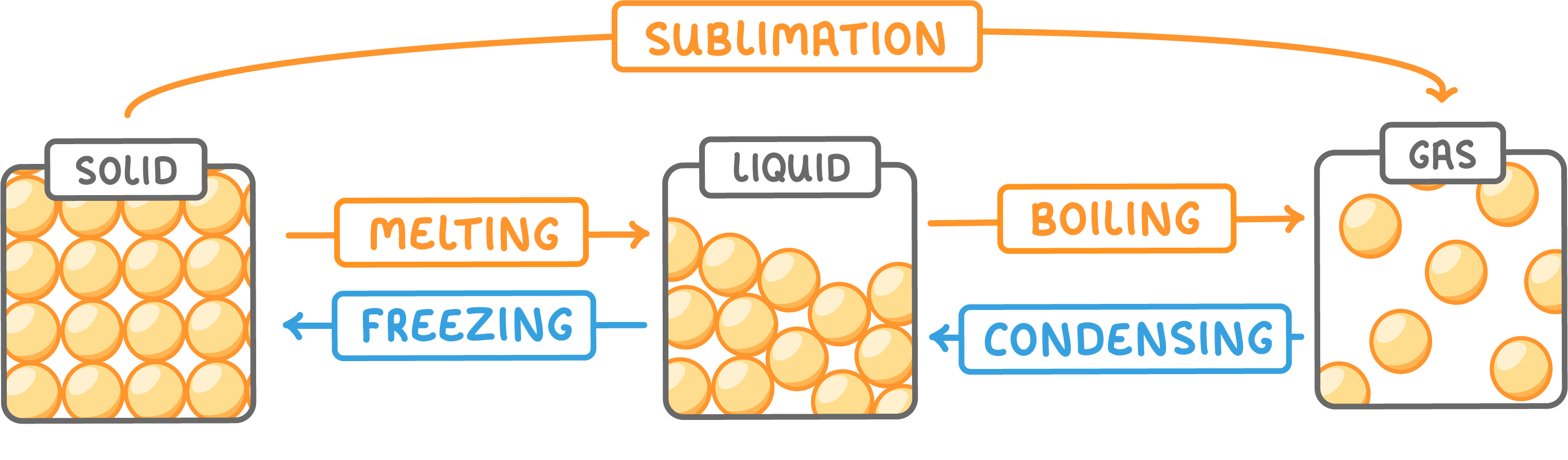
Changes of state describe the transition of matter from one state (solid, liquid or gas) into another state.
These transitions occur without altering the chemical composition of the substance.
Some substances can transition directly between the solid and gas states without entering the liquid phase.
This is called sublimation.
Heating causes particles to speed up
When a solid is heated:
- The particles gain kinetic energy and start vibrating more rapidly.
- This weakens the forces holding particles together so the solid expands.
- At the melting point, particles have enough energy to break free from fixed positions and the solid melts.
- The same happens when a liquid is heated and eventually reaches its boiling point, turning into a gas.
Cooling causes particles to slow down
When a gas is cooled:
- The particles lose kinetic energy and start moving more slowly.
- This strengthens forces between particles, pulling them closer together.
- At the condensation point, the particles have little energy and pack closer together, turning into a liquid.
- Further cooling condenses the liquid into a solid state at temperatures below the freezing point.
Density and state changes
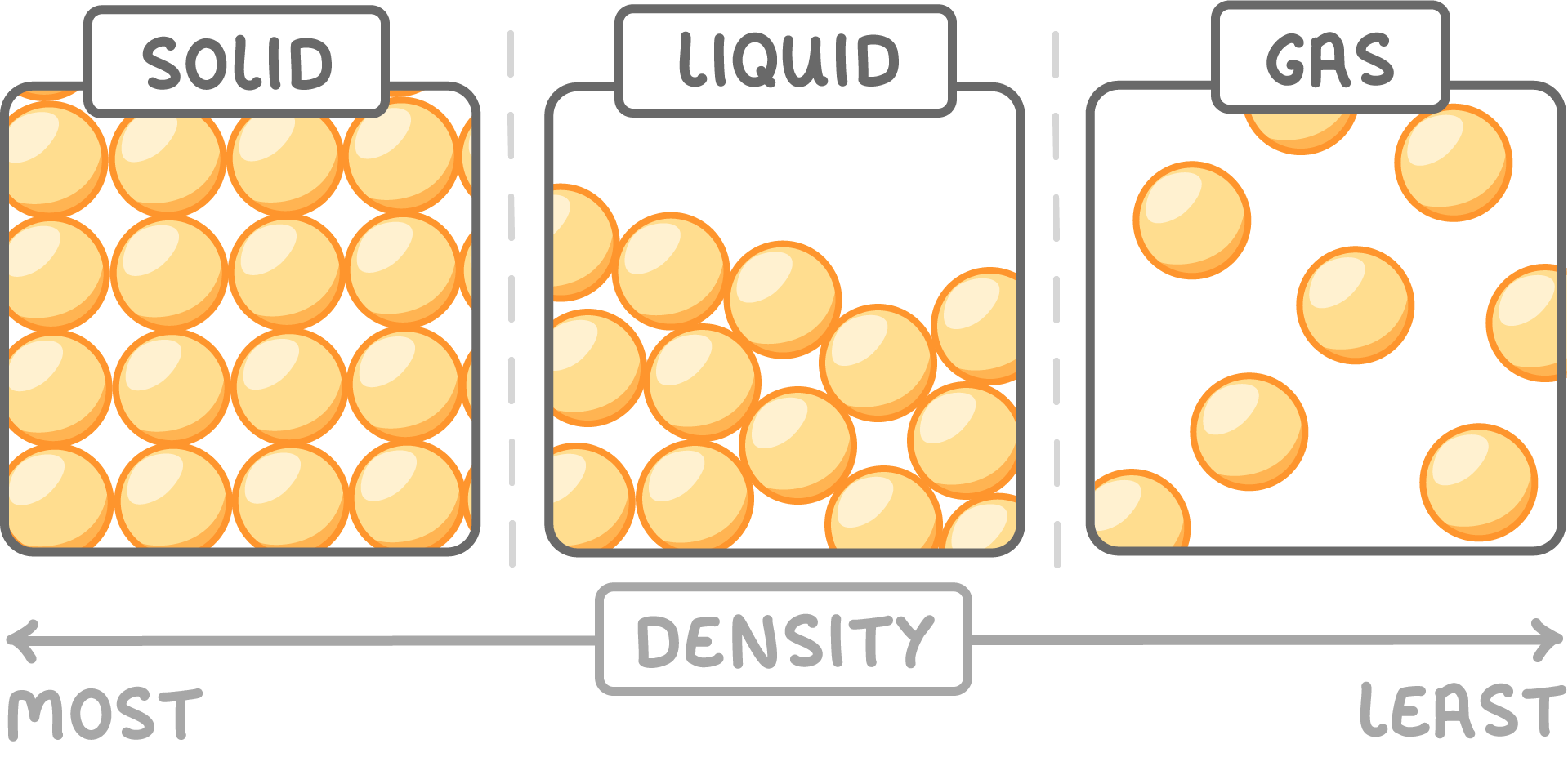
In most substances, density decreases during state change from a solid to a liquid and from a liquid to a gas.
However, water is an anomaly—ice is less dense than liquid water, allowing it to float.