Atoms and elements
This lesson covers:
- What an atom is
- What an element is
- Element names and symbols
What is an atom?
An atom is the basic building block of all matter.
They are extremely small and cannot be seen even with microscopes.
Dalton’s model of an atom
The first model of the atom was created John Dalton.
According to Dalton's model:
- All matter is made up of atoms.
- Atoms of different elements are different.
- Atoms of the same element are identical.
This explains why atoms of the same element always have the same properties. It is because they are identical.
What is an element?
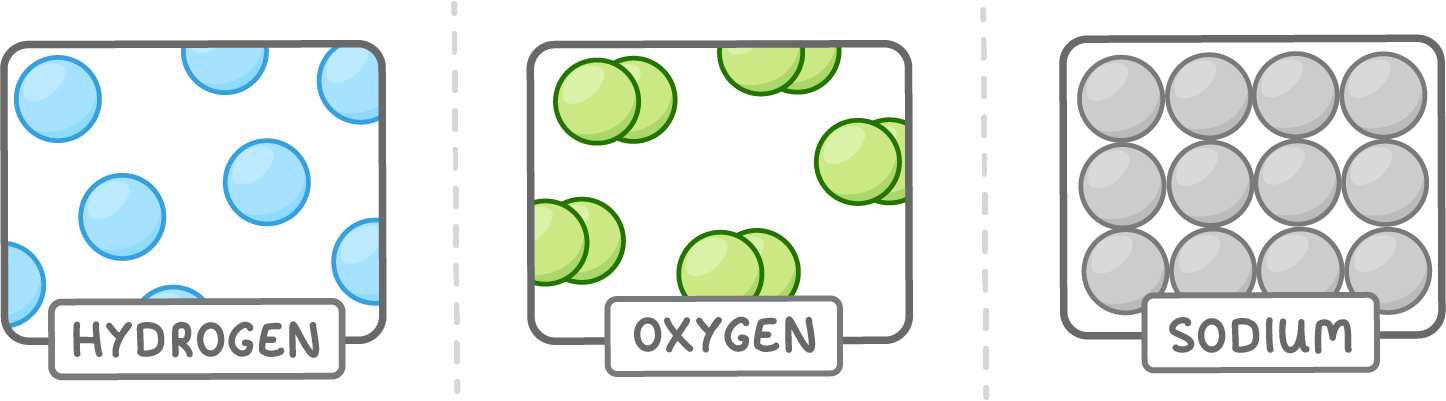
An element is a pure substance made up of only one type of atom.
Some examples of elements are:
- Hydrogen (H) - Is the lightest and most common element in the universe.
- Oxygen (O) - Is needed by all animals and plants to live.
- Sodium (Na) - Is a soft silvery metal that reacts violently with water.
There are about 100 different natural elements on Earth. Thousands more have been created artificially.
Element names and symbols
Each element has a unique name and a 1 or 2 letter symbol. You can find them all in the periodic table.
The first letter of the symbol is always capitalised. The second letter is lowercase.
Here are two examples:

- Name: Nitrogen
- Symbol: N

- Name: Lead
- Symbol: Pb