Examples of chemical reactions
This lesson covers:
- Combustion reactions
- Oxidation reactions
- Thermal decomposition
Combustion reactions

Combustion is basically burning. It happens when a fuel source reacts very rapidly with oxygen, giving off heat and light energy.
For example, a candle wax fuel burning, or a firework exploding.
The combustion reaction requires:
- Fuel (like oil, wood, gas)
- Heat to get it started
- Oxygen (usually from air)
When a fuel like methane burns in oxygen it makes carbon dioxide and water.
The energy released can provide useful heating and lighting.
Oxidation Reactions
Oxidation means a chemical reaction where a substance chemically combines with oxygen.
So combustion reactions are classed as oxidation since the fuel reacts with oxygen.
Another example of an oxidation reaction is iron rusting.
Here the iron metal reacts with oxygen gas in the air to form iron oxide (which is the chemical name for rust)
Thermal Decomposition
Some chemical substances actually break apart into other substances when you heat them up.
This is called thermal decomposition.
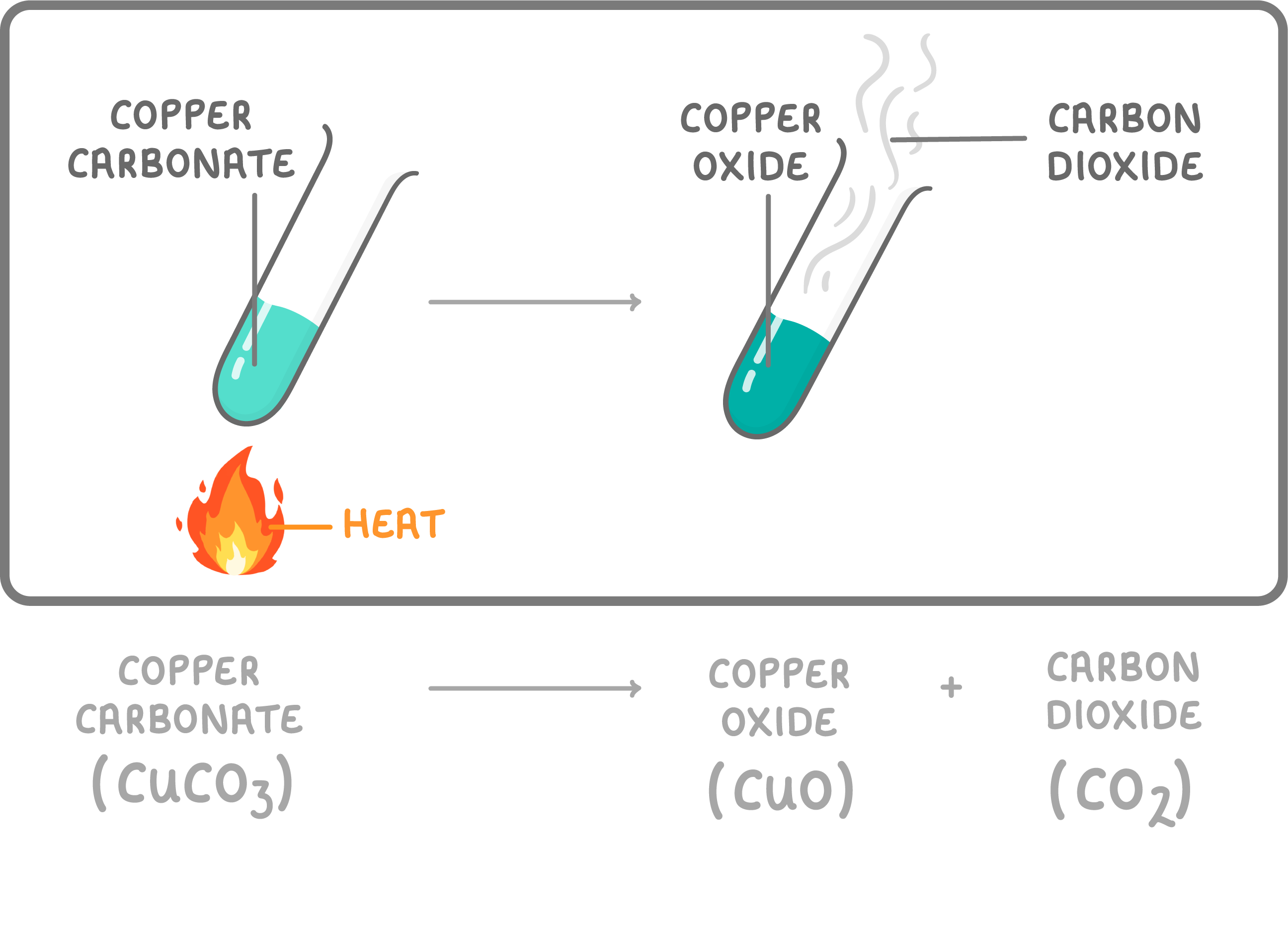
For example, a green compound called copper carbonate breaks down when heated into carbon dioxide gas and black copper oxide powder.