Factors Affecting Enzyme Activity
This lesson covers:
- What happens when an enzyme is denatured
- The effects of temperature and pH on enzyme-catalysed reactions
- The effects of enzyme and substrate concentration on enzyme-catalysed reactions
Enzyme denaturation Changes in temperature or pH can affect the rate of enzyme-catalysed reactions. |
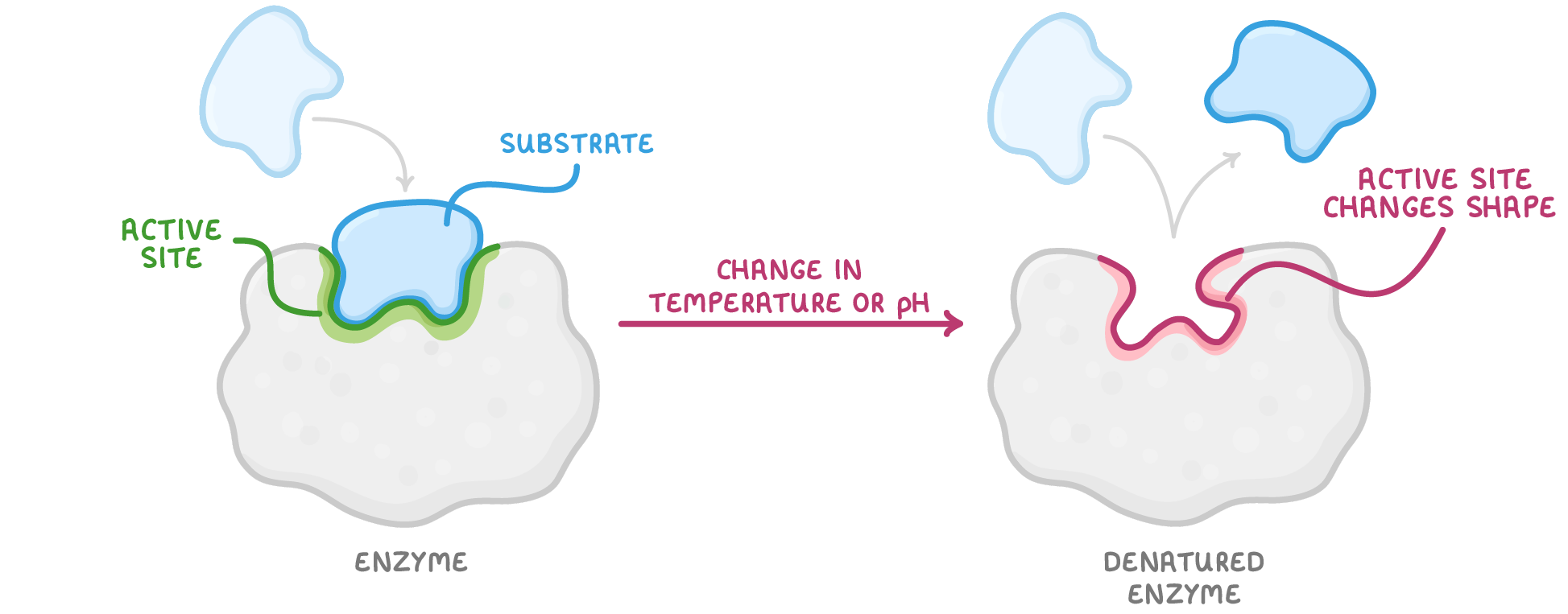 Drastic temperature increases or changes to the pH causes bonds to break, changing the enzyme's tertiary structure. This causes the active site to change shape so that the substrate no longer fits. This means that enzyme-substrate complexes cannot be formed and the enzyme is denatured. |
Different factors affect the rate of enzyme-controlled reactions You need to be able to describe and explain how the four factors affect enzyme reactions. These factors are:
|
Temperature All enzymes have an optimum temperature, but these can vary. The graph below shows how temperature affects the rate of a specific enzyme-catalysed reaction. 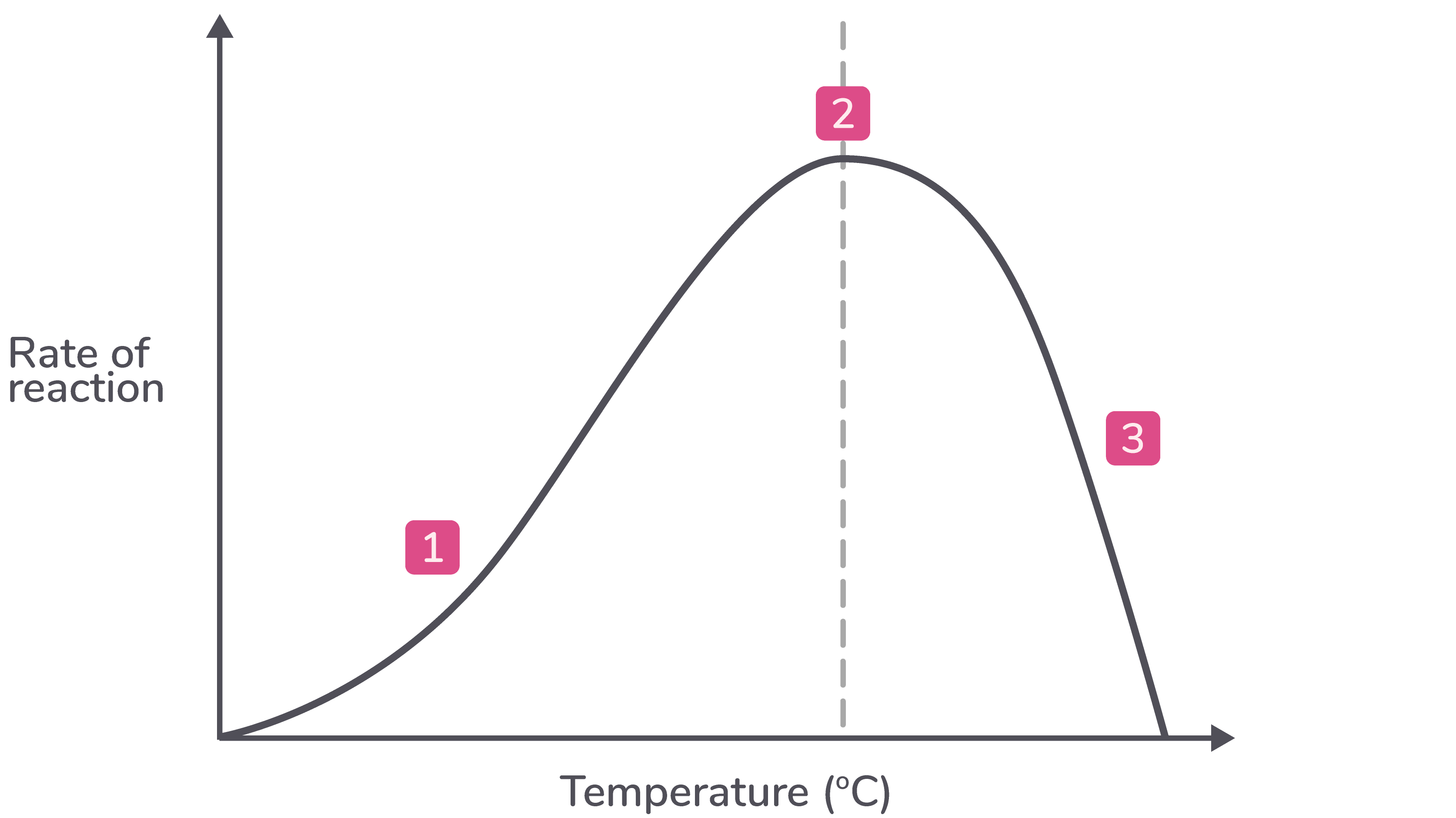 |
Explanation
Description
|
Temperature coefficient (Q10) The temperature coefficient (Q10) is a value that shows how much the rate of reaction changes when the temperature is increased by 10°C. It can be calculated using this formula: Q10=R1R2 Where:
|
pH All enzymes have an optimum pH, but these can vary. The graph below shows how pH affects the rate of a specific enzyme-catalysed reaction. 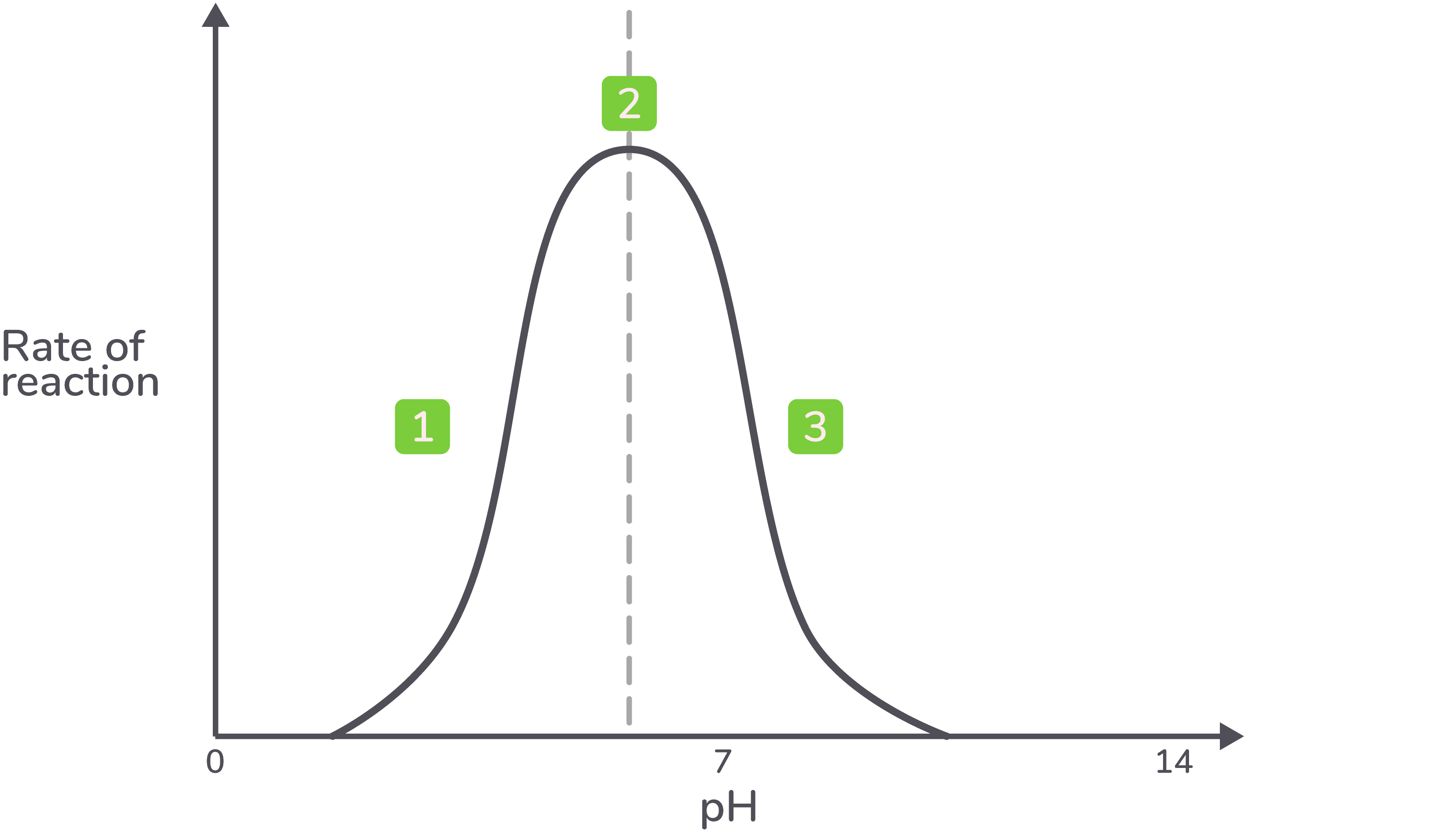 |
Explanation
Description
|
Substrate concentration The graph below shows how substrate concentration affects the rate of an enzyme-catalysed reaction. 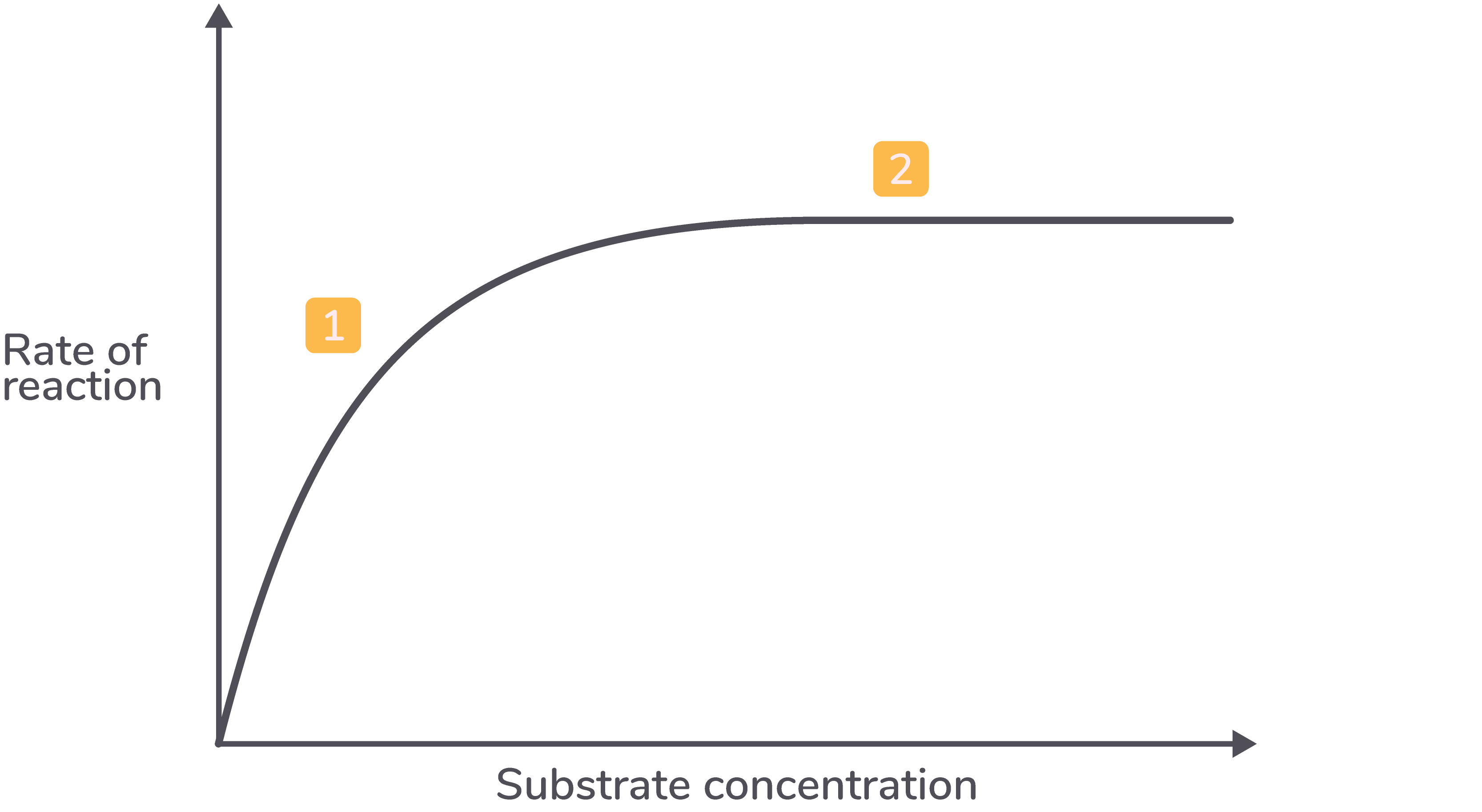 |
Explanation
Description
|
Enzyme concentration The graph below shows how enzyme concentration affects the rate of an enzyme-catalysed reaction.  |
Explanation
Description
|
Worked example - Calculating Q10
Given that at 20°C, the rate of reaction is 5 products produced per minute, and at 30°C, the rate of reaction is 10 products produced per minute, calculate Q10.
Step 1: Equation
Q10=R1R2
Step 2: Substitution and correct evaluation
Q10=510=2