Proteins: Globular & Fibrous Proteins
This lesson covers:
- The difference between globular and fibrous proteins
- How the structure of haemoglobin relates to its function
- How the structure of collagen relates to its function
In this lesson we have sometimes used tubes to represent polypeptides.
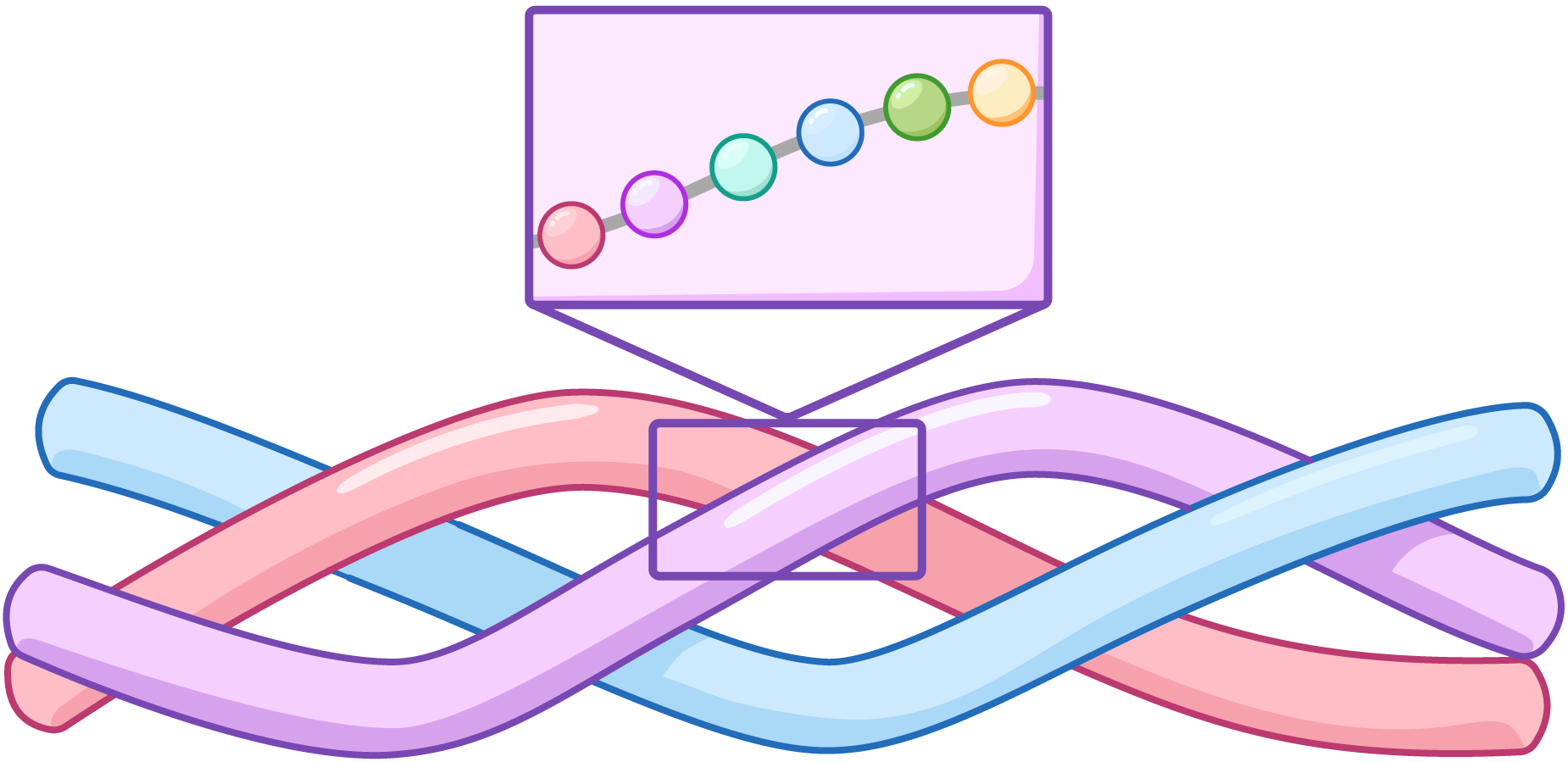
In reality these structures are complex, but for the purpose of understanding, each tube represents a separate polypeptide chain (a chain of amino acids bonded together by peptide bonds).
Types of proteins Proteins can be grouped into two categories: globular and fibrous proteins. |
Globular proteins Globular proteins are compact, spherical, and soluble proteins.  Globular proteins tend to have metabolic roles in the body, such as:
|
Fibrous proteins Fibrous proteins form long strands and are not usually soluble in water.  |
Fibrous proteins tend to have structural roles in the body, such as:
|
Collagen Collagen is an example of a fibrous protein used as a structural component in skin, tendons, cartilage, bones, teeth, and walls of blood vessels. Collagen is made up of three polypeptide chains, where each chain is twisted into a helix shape. The three chains are then wound around each other (like threads in a rope) to form a triple helix held together by hydrogen and covalent bonds. |
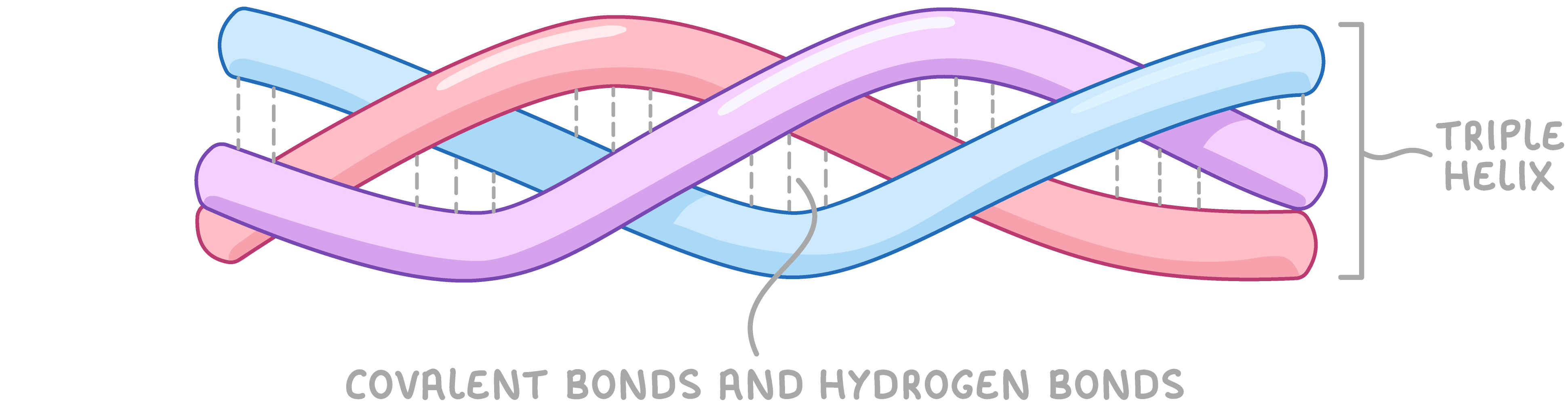 Within each chain, almost every third amino acid is that of glycine, the smallest amino acid. Its small size allows the three strands to be held closely together to form a tight coil. |
Collagen molecules, fibrils, and fibres 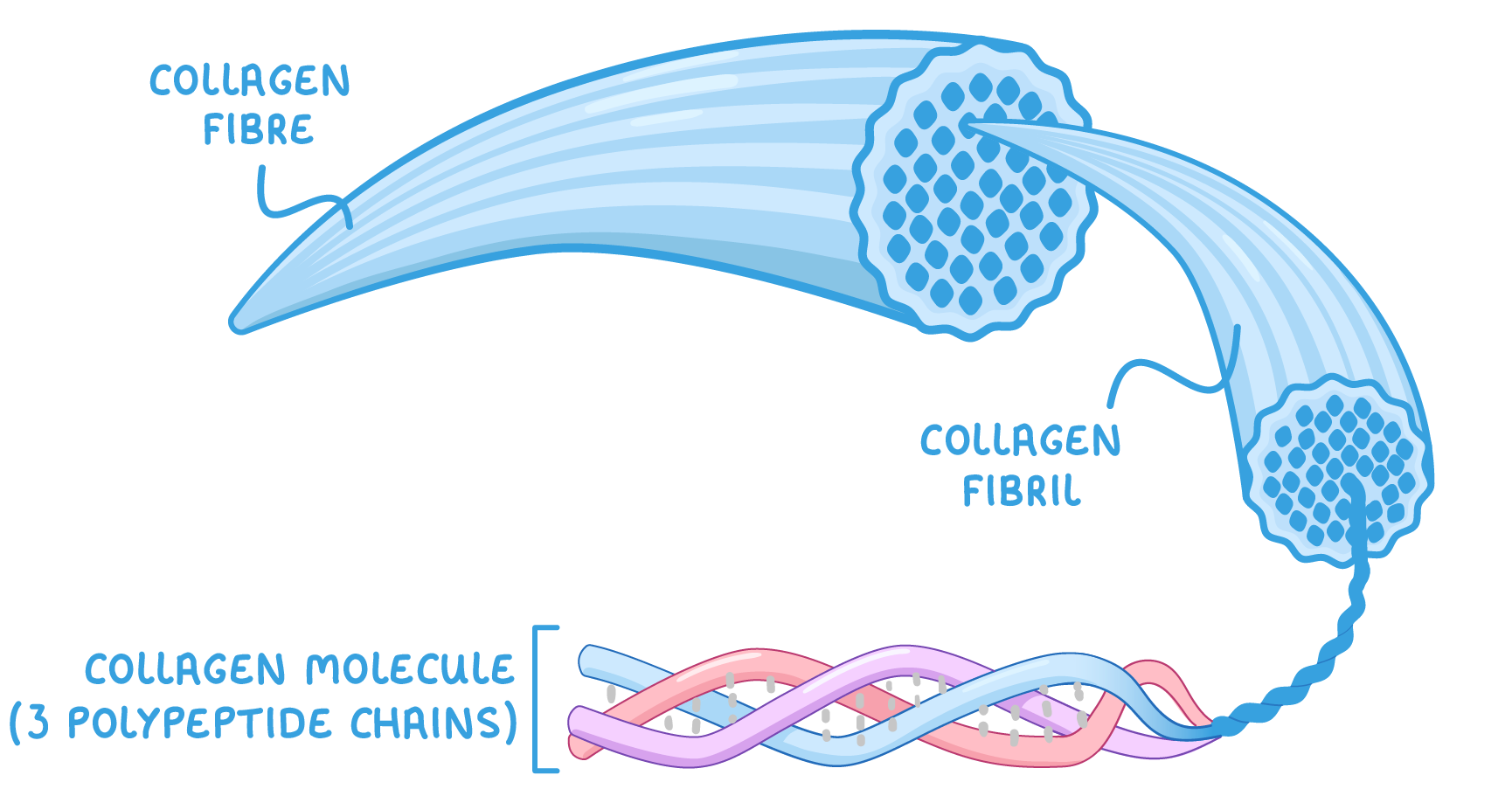 Collagen molecules (made up of three chains) lie parallel to one another where they form covalent bonds ('cross links') between the molecules. These cross links hold many collagen molecules together to form fibrils. The ends of the collagen molecules are staggered to prevent any weak spots. Many fibrils lie alongside each other to form strong bundles known as fibres. |
Adaptations of collagen for its role The structure of collagen is well adapted for its role:
|
Haemoglobin Haemoglobin is an example of a globular protein used to carry oxygen around the body in red blood cells. It is made up of four polypeptide chains, meaning it has a quaternary structure. Two of the polypeptide chains (α chains) are made from a protein known as alpha-globin, whereas the other two chains (β chains) are made from a protein known as beta-globin. |
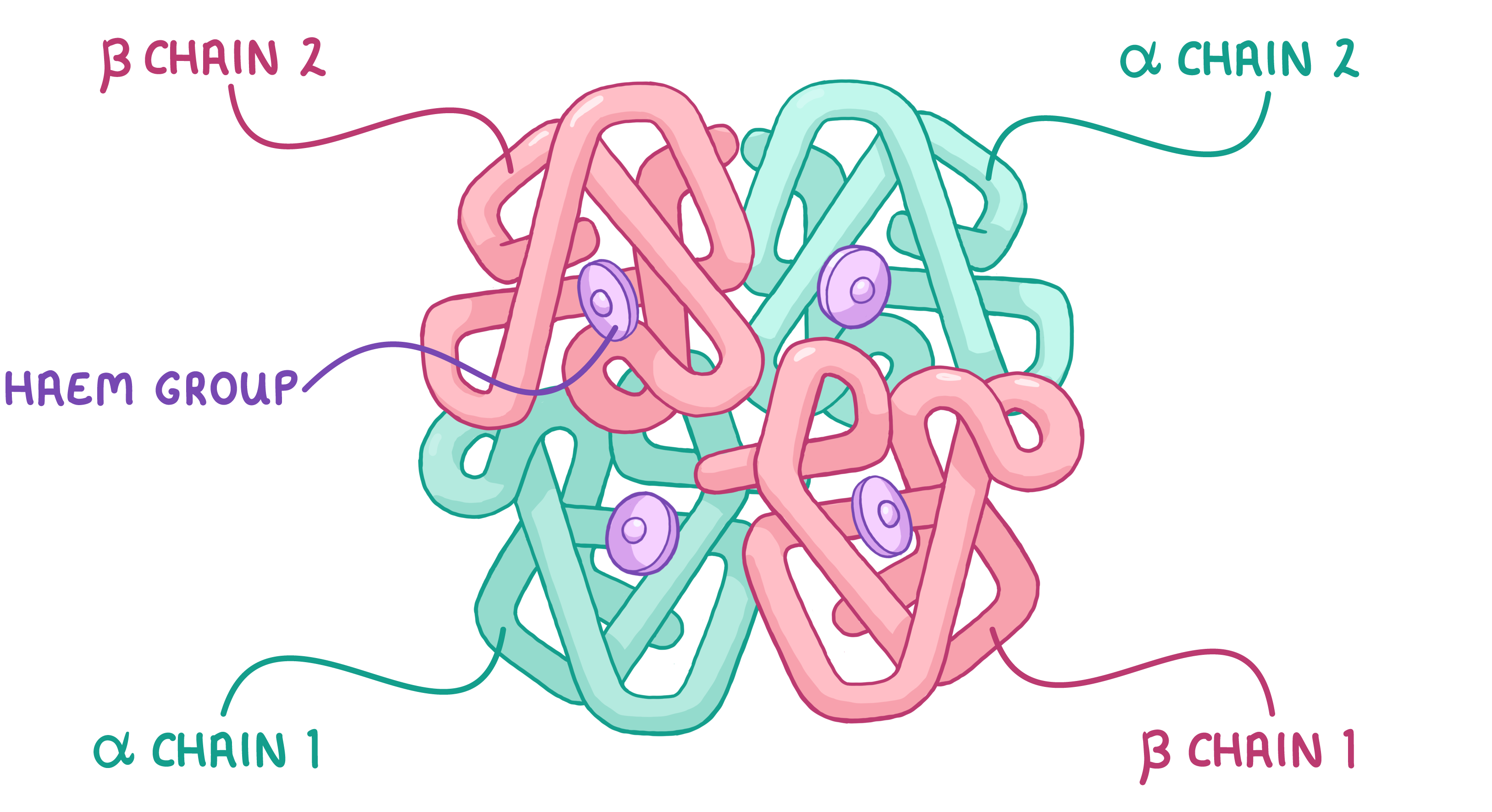 |
Each polypeptide chain contains a haem group (prosthetic group) which contains an iron ion. Each iron ion can reversibly bind with one oxygen molecule (O2). This means that a molecule of haemoglobin (with four haem groups) can carry four oxygen molecules at a time. |
The following features allow haemoglobin to transport oxygen around the body:
|