Proteins: Amino Acids
This lesson covers:
- The roles of proteins in living organisms
- The structure of amino acids
- The synthesis and breakdown of peptide bonds
- How to test for proteins
Proteins are made up of amino acids Amino acids are the building blocks of proteins, which are essential macromolecules involved in various functions within living organisms. Amino acids are monomers and can join together via peptide bonds to form dimers (dipeptides) and polymers (polypeptides). 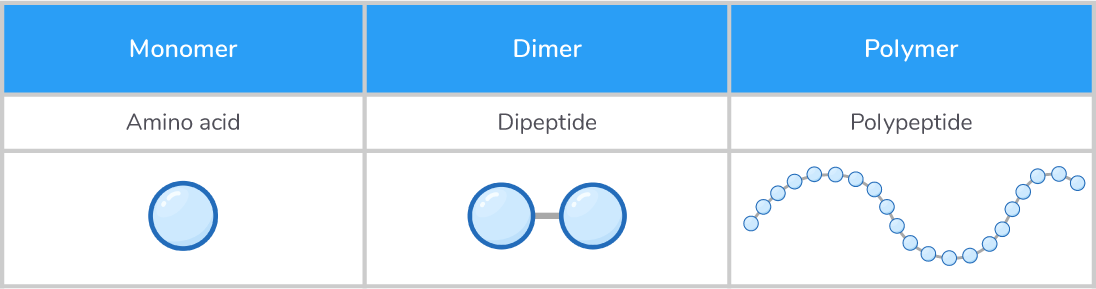 |
What are the roles of proteins? Functions of proteins in living organisms:
|
Amino acid structure There are around 20 different amino acids that are commonly found in living organisms. 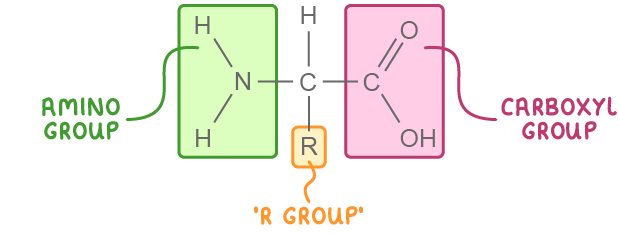 They all have the same general structure:
Each amino acid has a different R group which determines its properties. For example, amino acid cysteine contains a sulphur atom in its R group. This allows cysteine to form disulphide bonds. |
Dipeptide synthesis and breakdown Dipeptides are synthesised via condensation reactions and broken down via hydrolysis reactions. These reactions involve the formation or the breakdown of a covalent bond known as a peptide bond. |
Condensation reaction 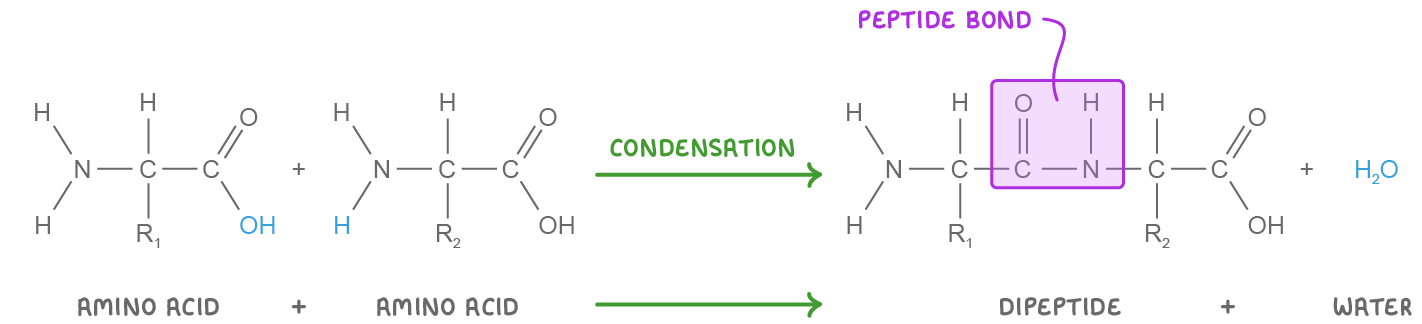 When two amino acids join, the hydroxyl (OH) in the carboxyl group of one amino acid reacts with the hydrogen (H) in the amino group of another amino acid. This releases a water molecule (H2O) and forms a peptide bond between the carbon of one amino acid and the nitrogen of another. |
Hydrolysis reaction 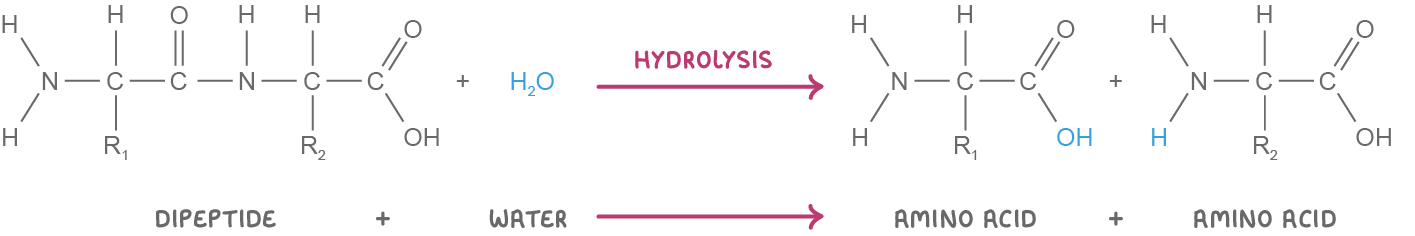 When a water molecule (H2O) is added to a dipeptide, the peptide bond is broken to release the two amino acids. |
Testing for proteins
To find out whether a sample contains peptide bonds (and hence, proteins), you must carry out the Biuret test.
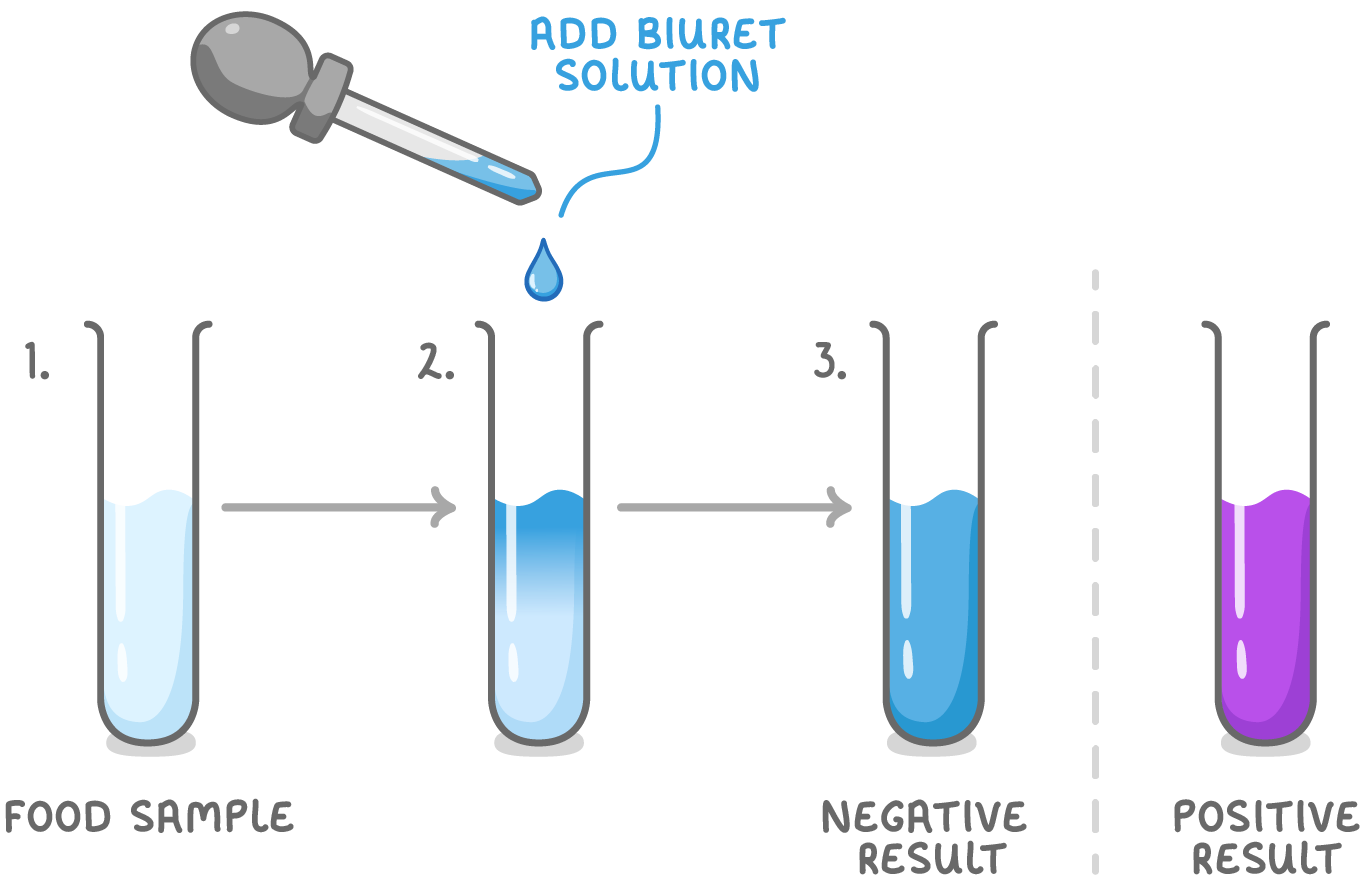
Steps to find out whether a sample contains proteins:
- Place your food sample in a test tube.
- Add an equal volume of Biuret solution (sodium hydroxide and copper sulfate).
- If proteins are present, the solution will turn from blue to purple. If no protein is present, the solution remains blue.