Carbohydrates: Monosaccharides & Disaccharides
This lesson covers:
- The different types of monosaccharides
- The difference between alpha and beta glucose
- The different types of disaccharides
- The reactions which form and break down disaccharides
Monosaccharides Monosaccharides are the simplest form of carbohydrates, also known as 'simple sugars'. Monosaccharides are soluble, sweet-tasting and are found in many foods such as fruits, vegetables, and grains. They have the general formula (CH2O)n where 'n' can be any number from 3 to 7. |
Monosaccharides are classified according to the number of carbon atoms in each molecule: Hexose sugars (6 carbon atoms)
Pentose sugars (5 carbon atoms)
|
Alpha-glucose and beta-glucose Glucose is a hexose (6-carbon) sugar with the formula C6H12O6. The atoms in glucose can be arranged in two different ways. This means that there are two isomers of glucose:
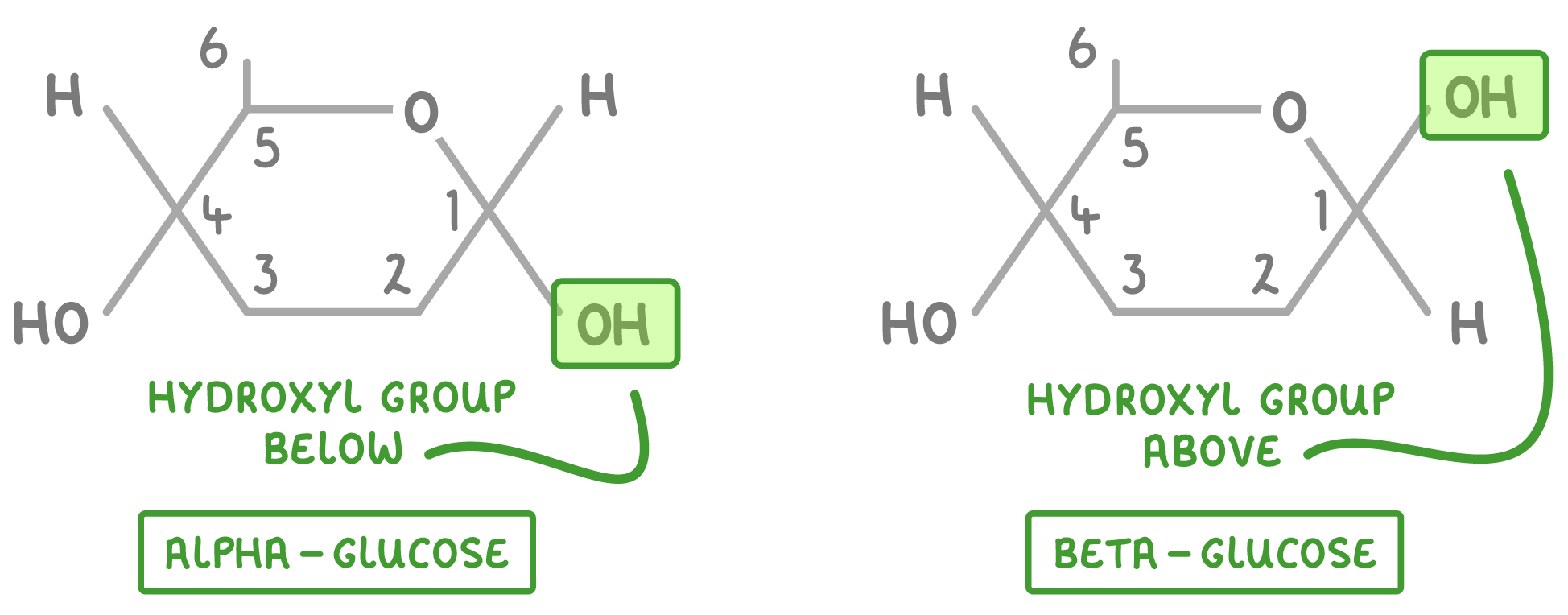 The only difference between the two forms is the orientation of the hydroxyl group (OH) on carbon 1 (the first carbon atom in the ring). |
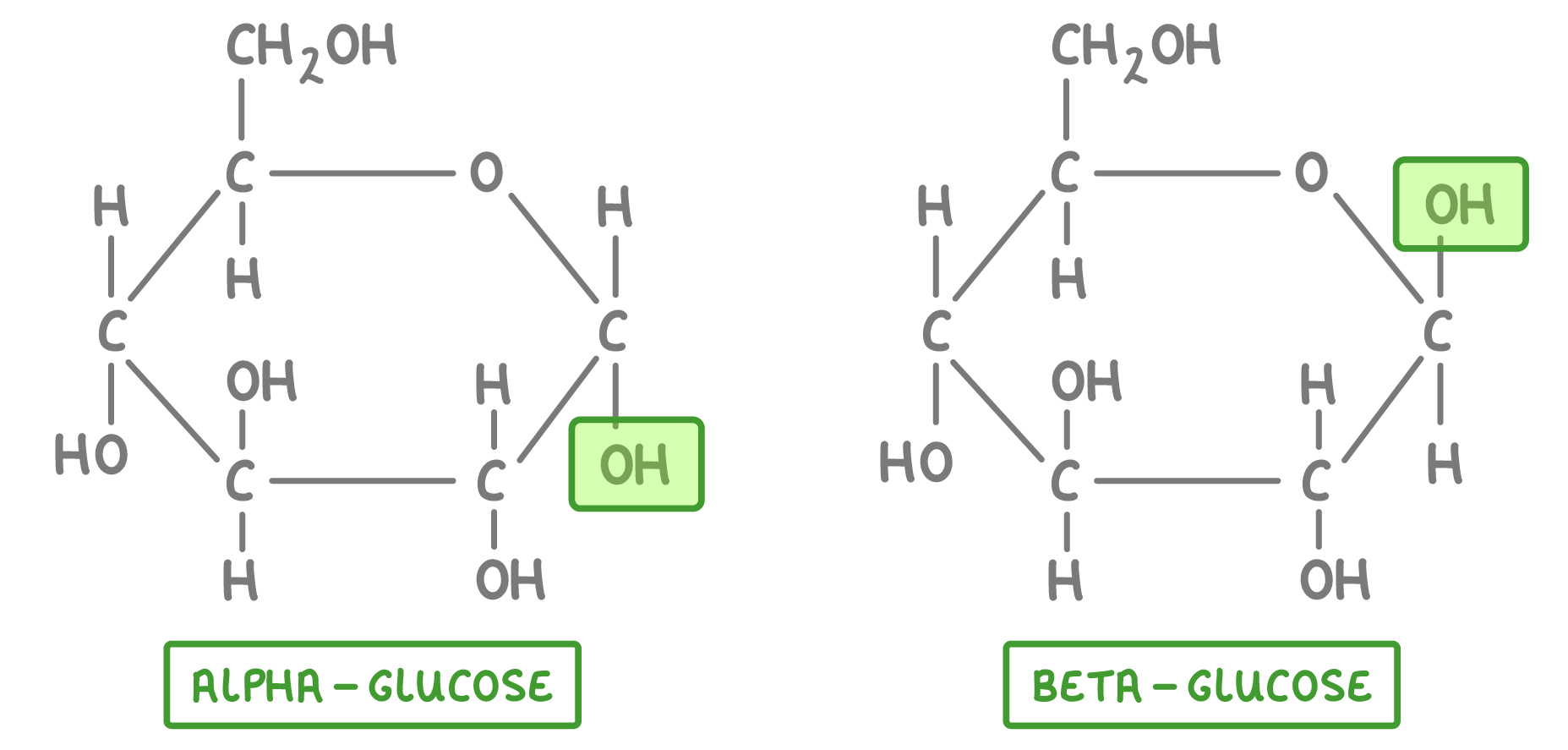 This diagram is showing the same molecules, but shows all the atoms. |
Properties and uses of glucose Glucose is used as the primary energy source in animals and plants. The following features of glucose help it to function as an energy source:
|
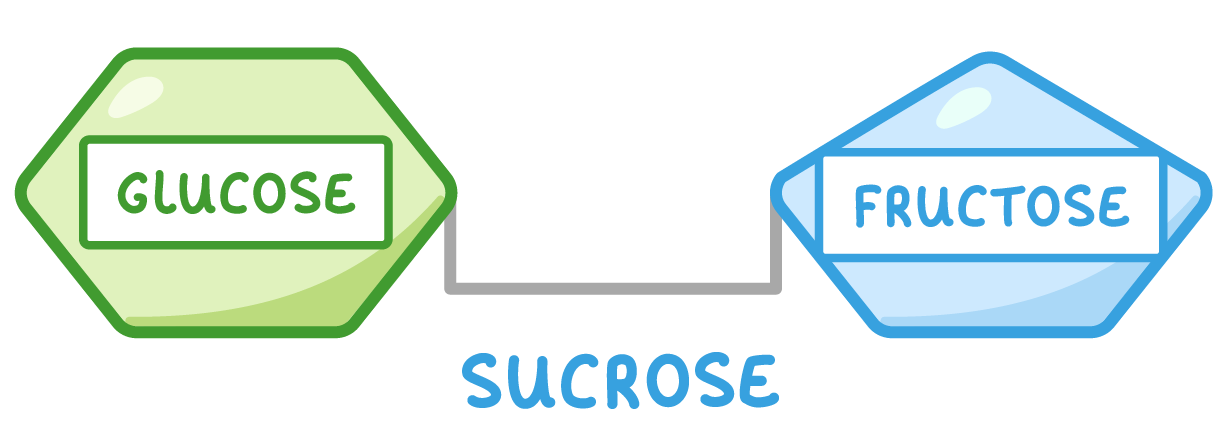
Disaccharides Disaccharides are formed when two monosaccharides join together. Examples of disaccharides include maltose (found in grains and cereals), sucrose (used as a transport sugar in plants), and lactose (the main carbohydrate found in milk). 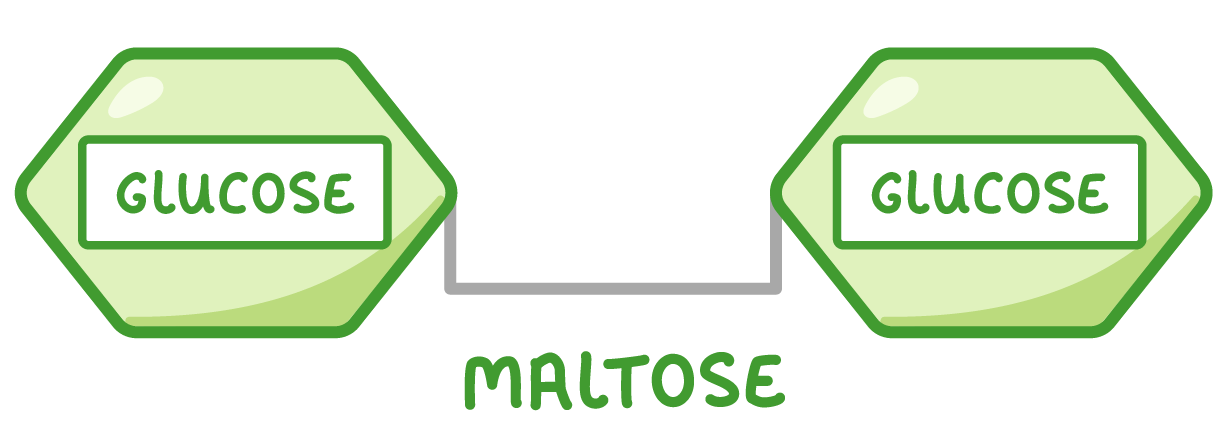
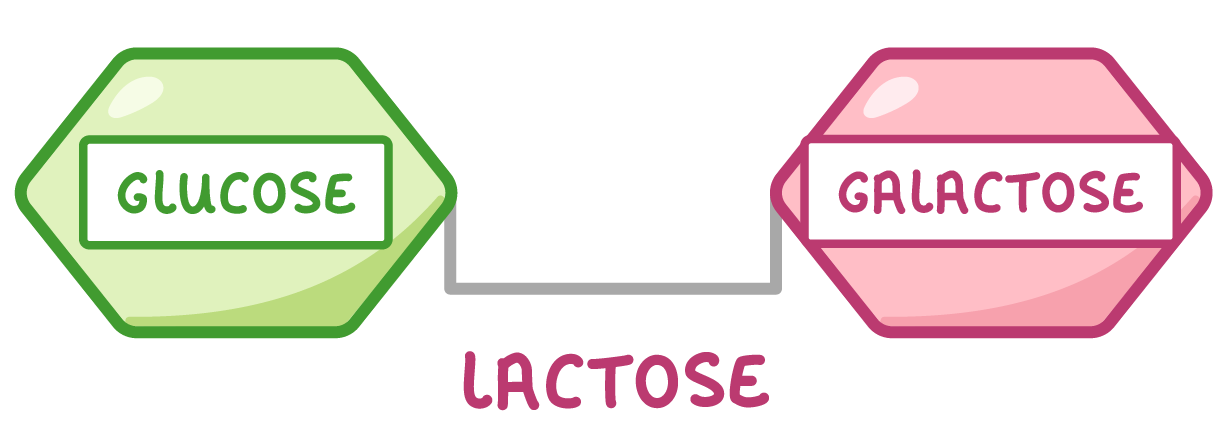
|
Disaccharide formation and breakdown Disaccharides are created via condensation reactions, and broken down via hydrolysis reactions. These reactions involve the formation or the breakdown of a covalent bond known as a glycosidic bond. |
Condensation reaction 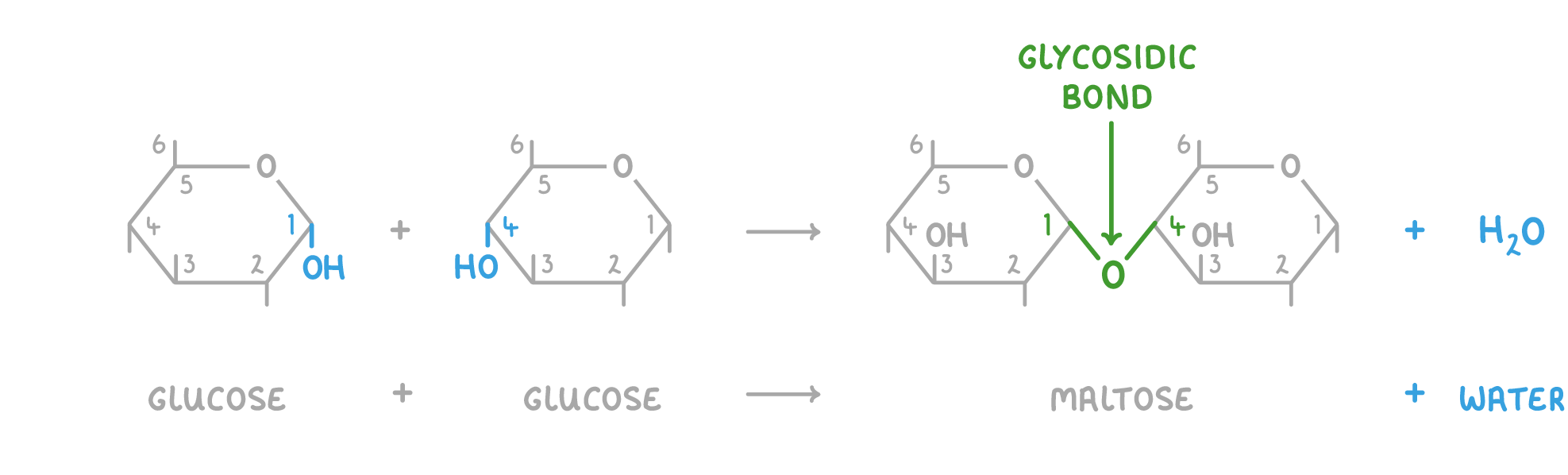 When two monosaccharides join, the hydroxyl group (OH) on carbon 1 of one monosaccharide reacts with the hydroxyl group (OH) on carbon 4 of another monosaccharide. A 1-4 glycosidic bond is formed and a water molecule (H2O) is released. |
Hydrolysis reaction 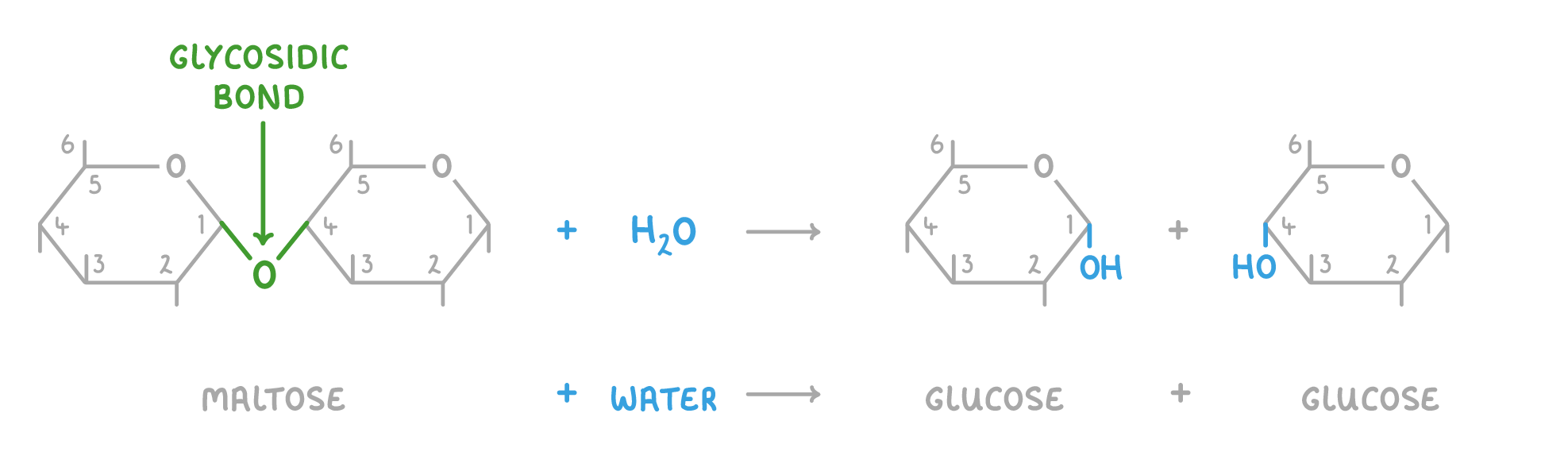 When a water molecule (H2O) is added to a disaccharide, the glycosidic bond is broken to release the 2 monosaccharides. |