Comparing Enzyme Affinities
This lesson covers:
- What enzyme affinity means
- The definition of Vmax
- How to derive Km from a curve
Enzyme affinity When comparing enzymes, we often talk about the affinity of each enzyme. Here, affinity refers to the strength of the attraction between an enzyme and its substrate:
|
Vmax
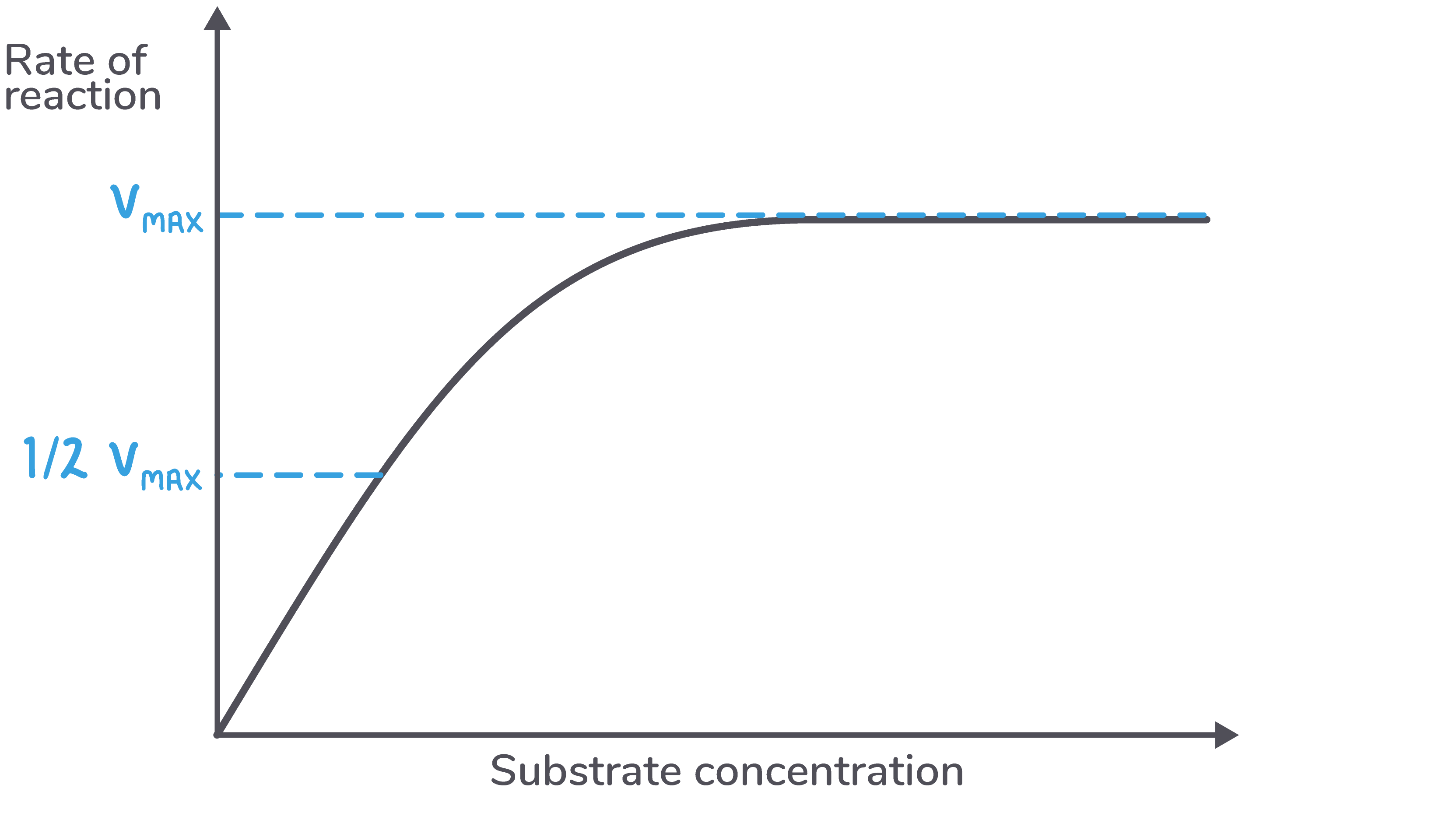
Increasing the substrate concentration increases the rate of an enzyme-catalysed reaction, but only up to a certain point. Eventually a saturation point is reached, where all active sites are occupied by a substrate.
This maximum rate of reaction is known as Vmax.
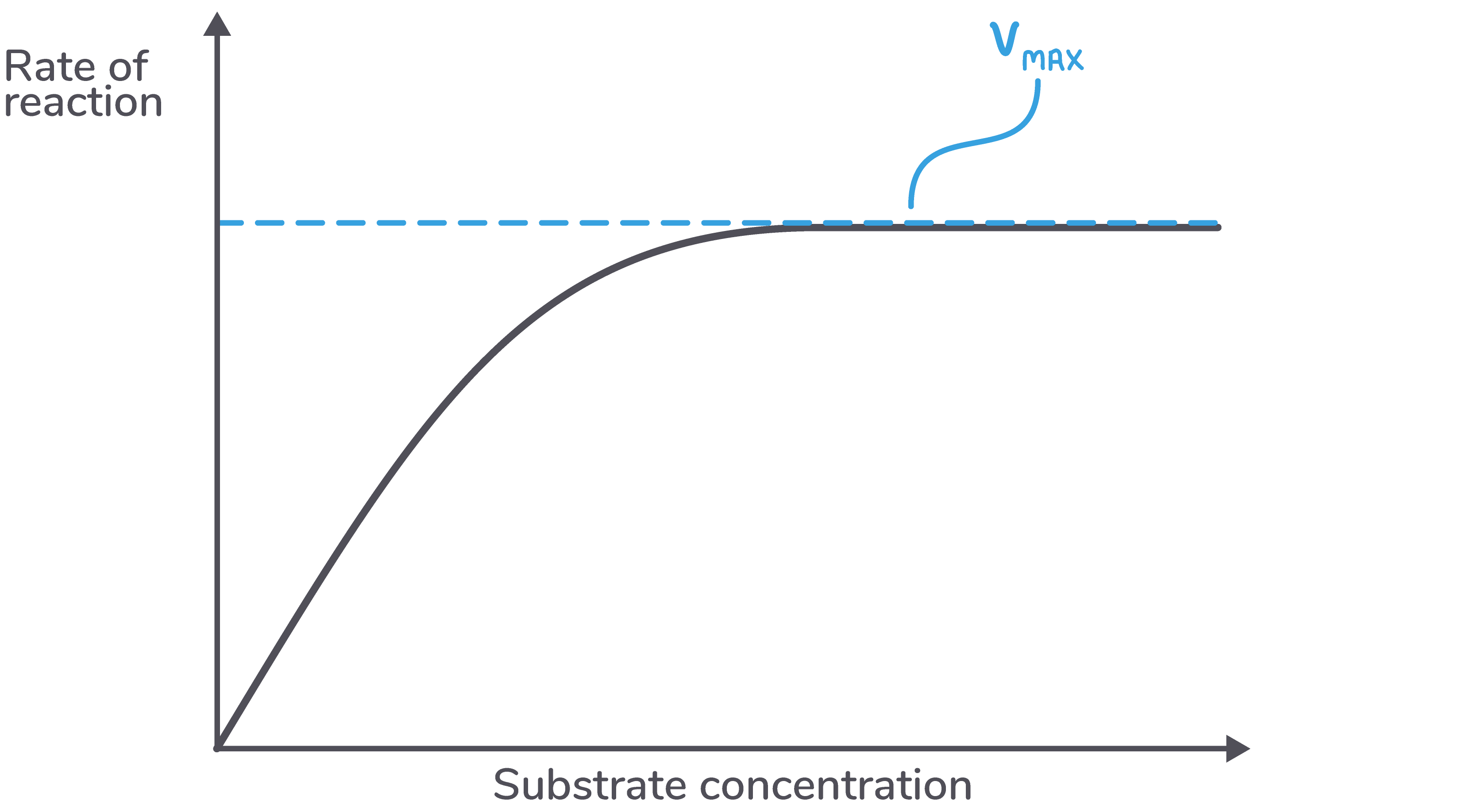
Vmax can be used as an indicator for the efficiency of an enzyme. However, the type of curve shown above never flattens out in practice, only in theory.
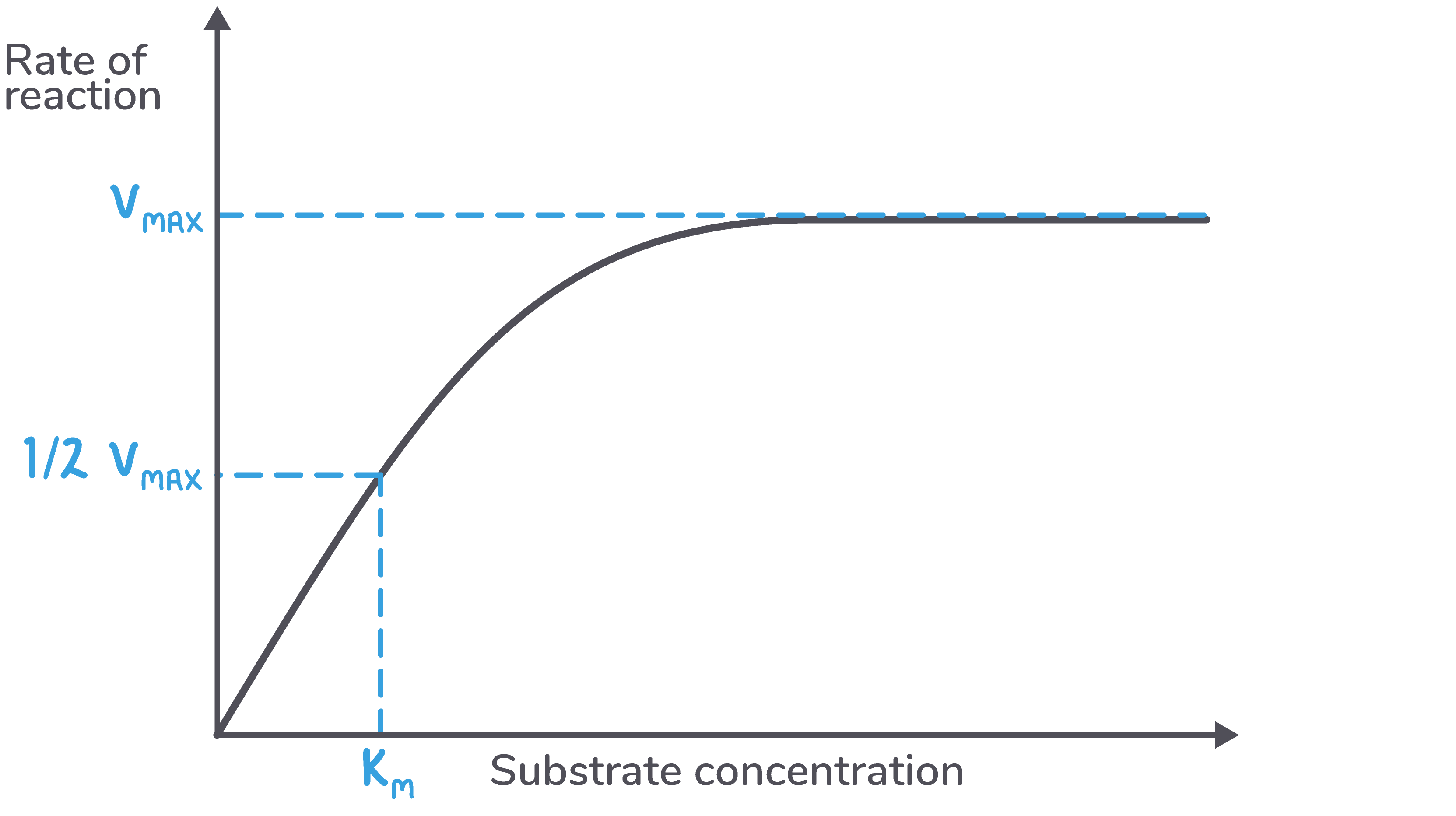
Michaelis-Menten constant (Km) Instead of using Vmax, we can use a value known as Km to measure the affinity of an enzyme for its substrate. Km is the substrate concentration at which half the enzyme's active sites are bound to a substrate. |
We can calculate Km from a graph by following these three steps: 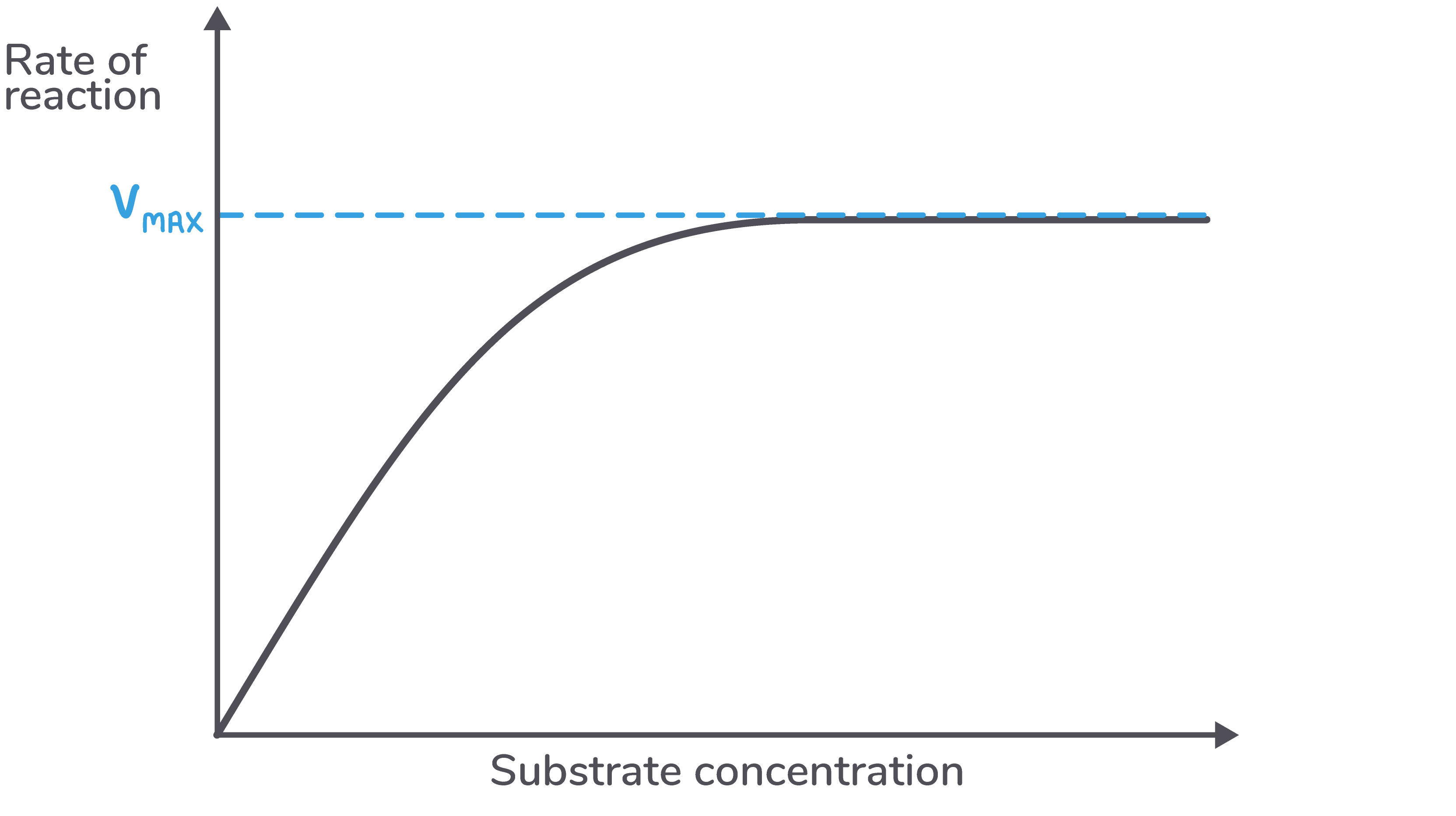 Calculate Vmax from the curve - This is the maximum rate. |
Interpreting Km values The higher the affinity of an enzyme for its substrate, the lower the substrate concentration needed to reach Vmax. Therefore, the higher the affinity of an enzyme for its substrate, the lower its Km value will be. |