Enzyme Inhibition
This lesson covers:
- The different types of inhibitors
- How competitive inhibitors affect enzyme action
- How non-competitive inhibitors affect enzyme action
Types of inhibitors Inhibitors are molecules that bind to enzymes to reduce their activity. The effects of inhibitors can be reversible or irreversible:
|
Inhibitors can be grouped into two categories:
These inhibitors will be covered in more detail later in this lesson. |
Competitive inhibitors Competitive inhibitors bind to the active site of an enzyme to prevent enzyme-substrate complexes. |
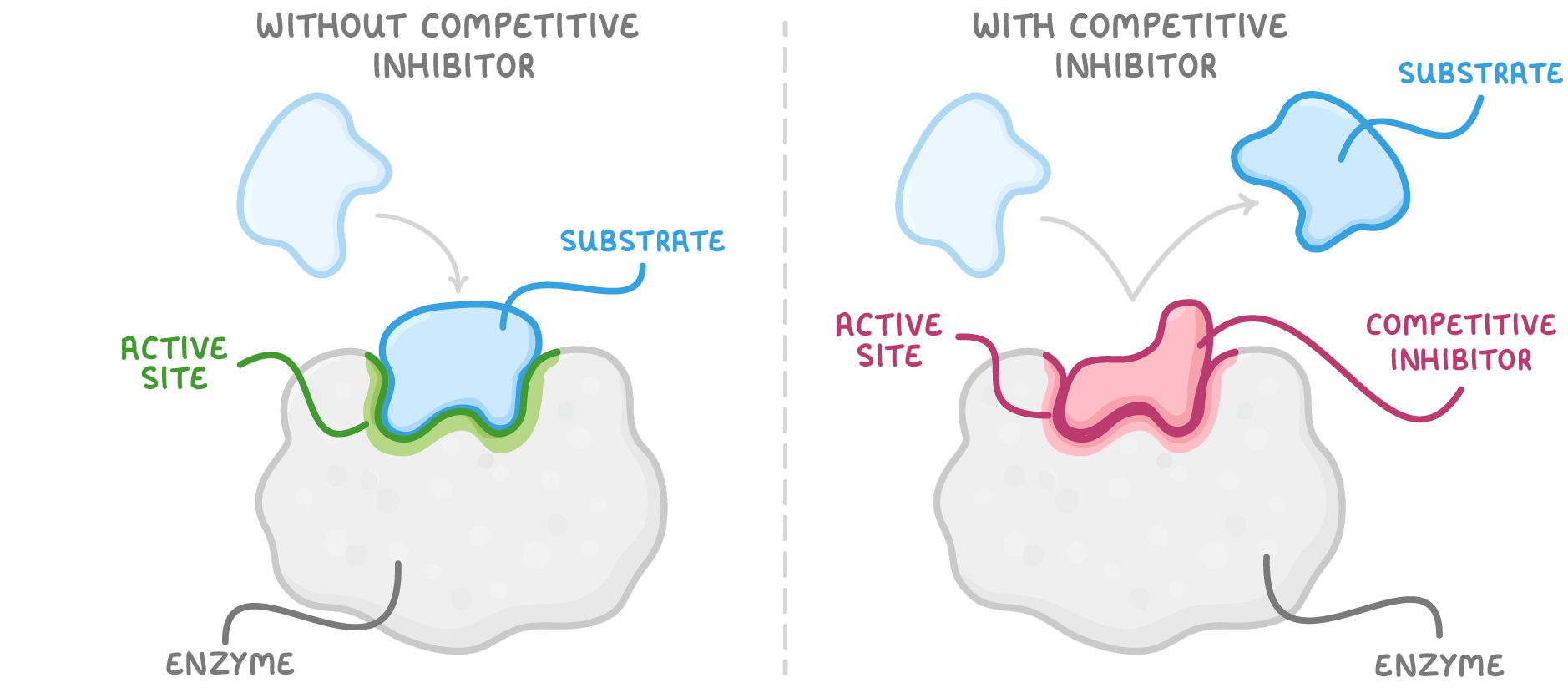 Competitive inhibitors have a similar shape to the substrate and so they bind to the active site of the enzyme. This prevents the substrate from binding, and thus reduces the formation of enzyme-substrate complexes. This results in a decrease in the rate of the enzyme-catalysed reaction. Most competitive inhibitors are reversible as they only temporarily bind to the enzyme. |
Increasing substrate concentration increases rate of reaction Competitive inhibitors can be overcome by increasing the substrate concentration. 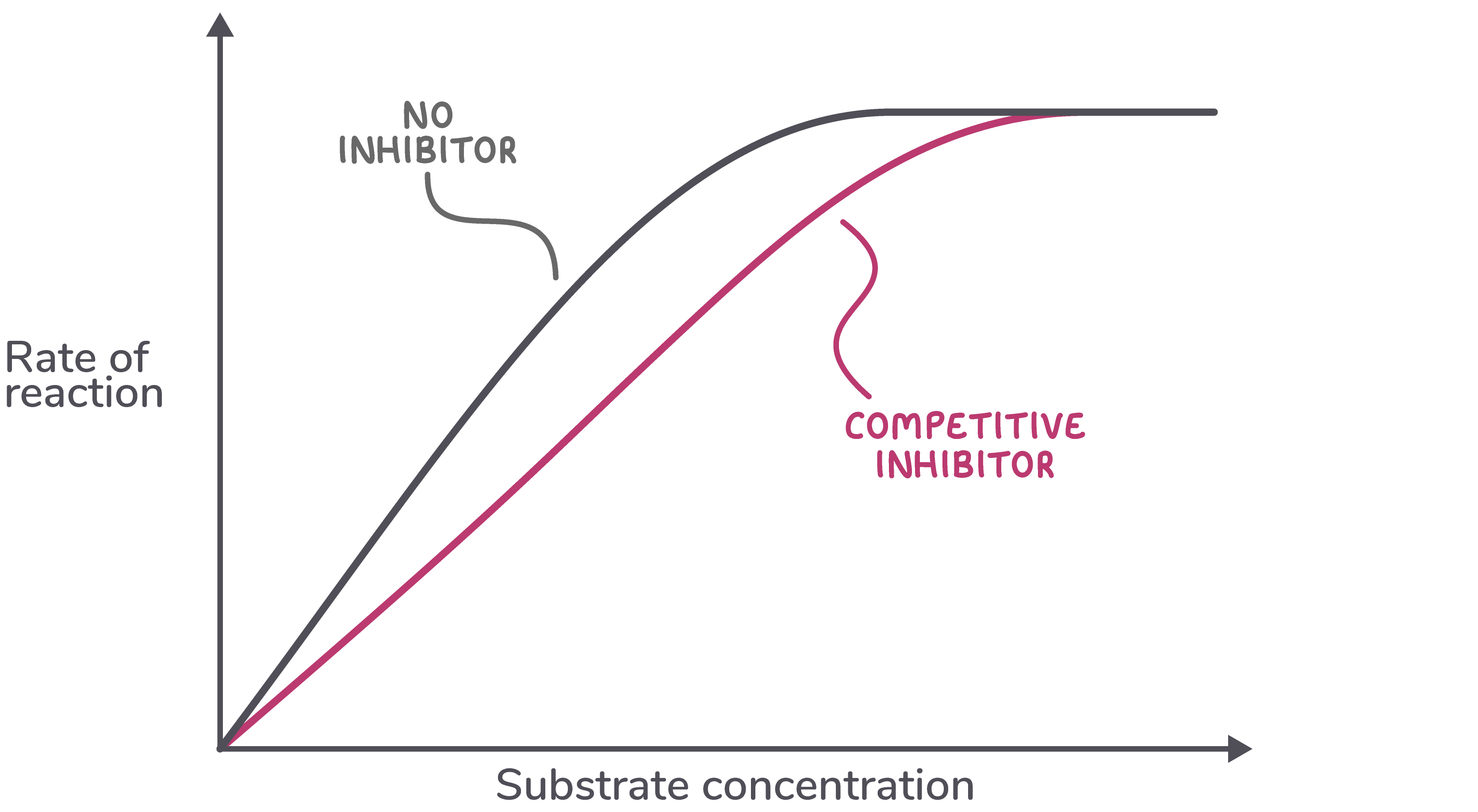 |
The higher the substrate concentration, the more likely it is that substrates will bind to active sites rather than inhibitor molecules. This will reduce the effect of the competitive inhibitor. |
Non-competitive inhibitors Non-competitive inhibitors bind to enzymes away from the active site (allosteric site) to prevent enzyme-substrate complexes. |
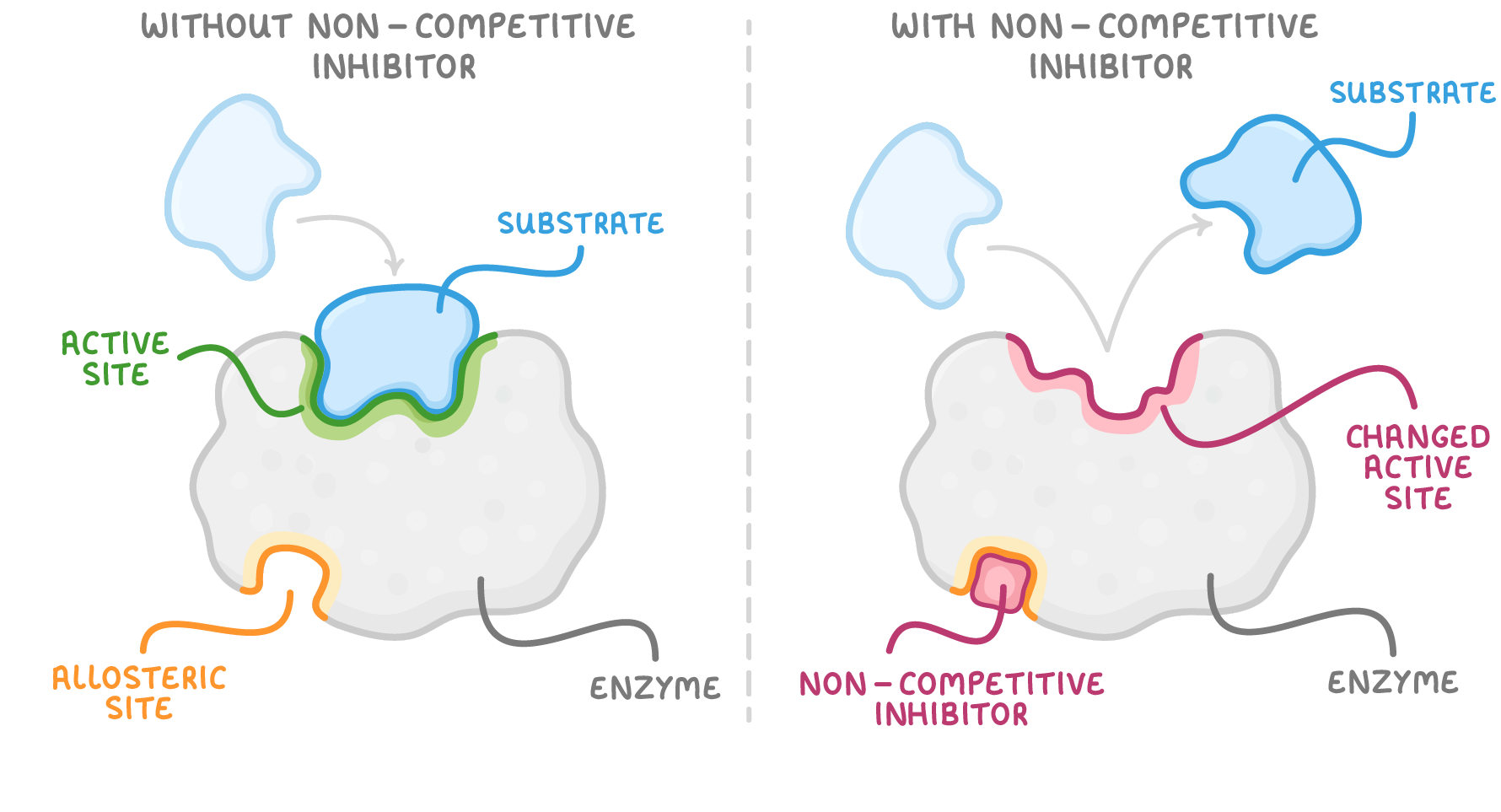 This binding changes the tertiary structure of the enzyme, causing the active site to change shape. This results in the active site no longer being complementary to the substrate, thus the substrate and enzyme cannot bind. Less enzyme-substrate complexes are formed and the rate of the enzyme-catalysed reaction decreases. |
Increasing substrate concentration has no effect on the rate of reaction Non-competitive inhibitors cannot be overcome by increasing substrate concentration. 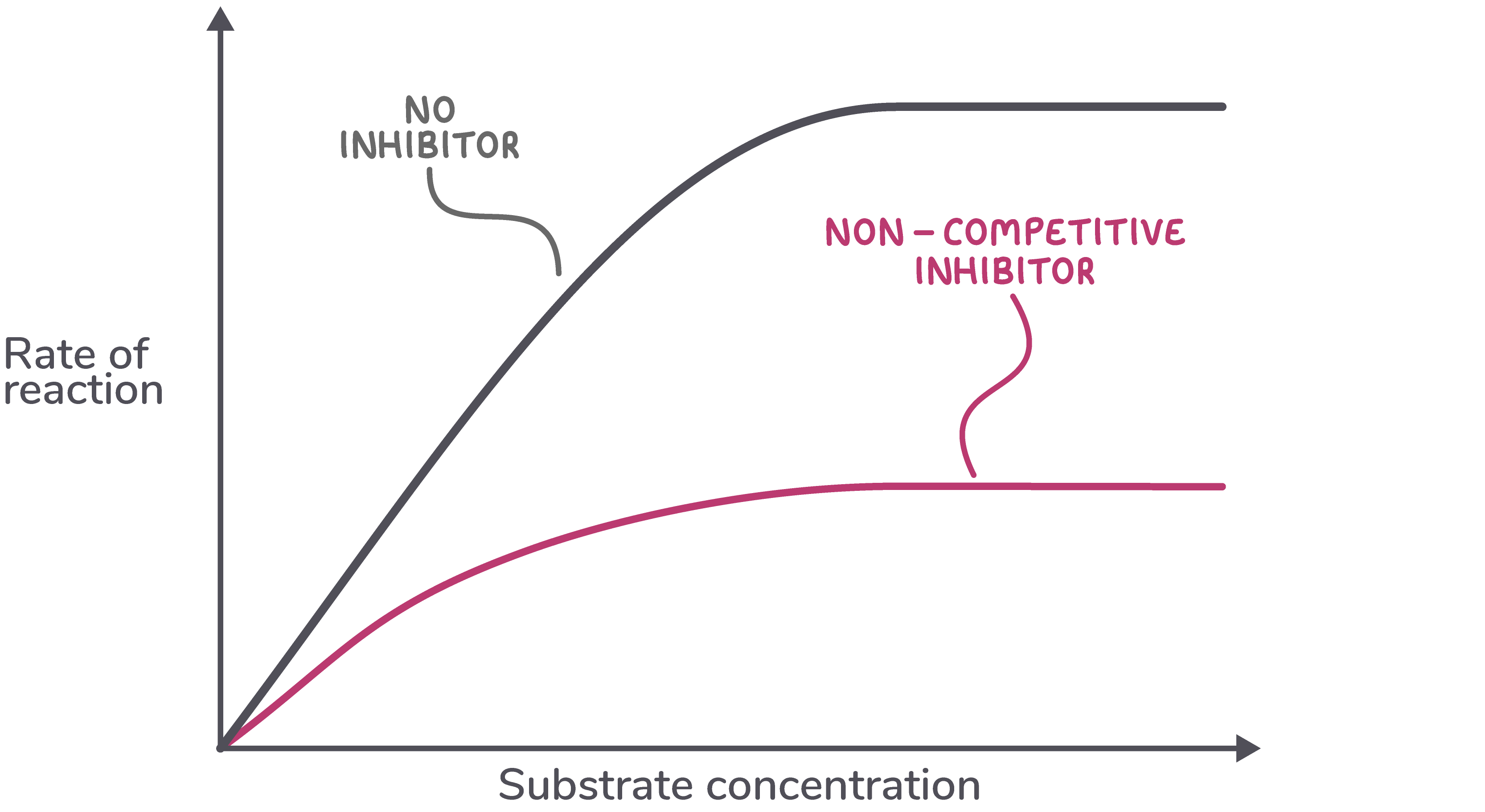 |
Non-competitive inhibitors do not compete with the substrate to bind to the active site, so increasing the amount of substrate has no effect on the rate of reaction. |