Water
This lesson covers:
- The structure of water
- Hydrogen bonding in water
- The role of water in living organisms
- How the properties of water relate to its function
Structure of water A molecule of water (H2O) is made up of one oxygen atom (O) joined to two hydrogen atoms (H). These atoms are held together by two covalent bonds. 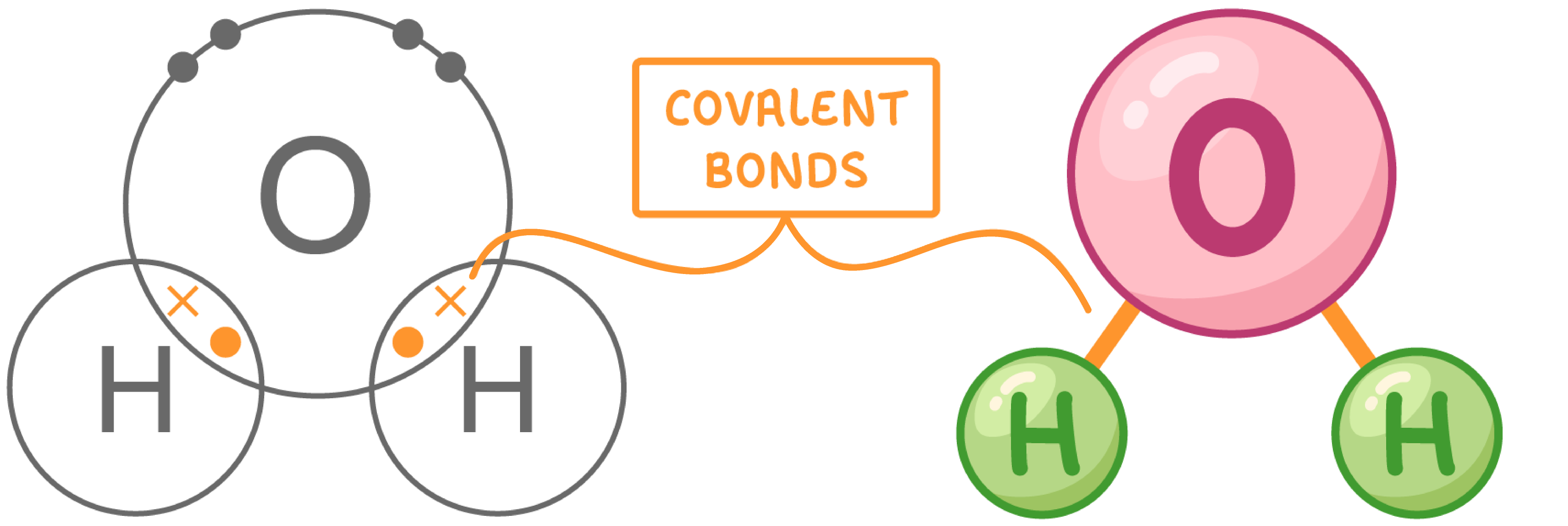 Oxygen shares one electron with each hydrogen atom while each hydrogen atom shares its one electron with oxygen. |
Water is a dipolar molecule 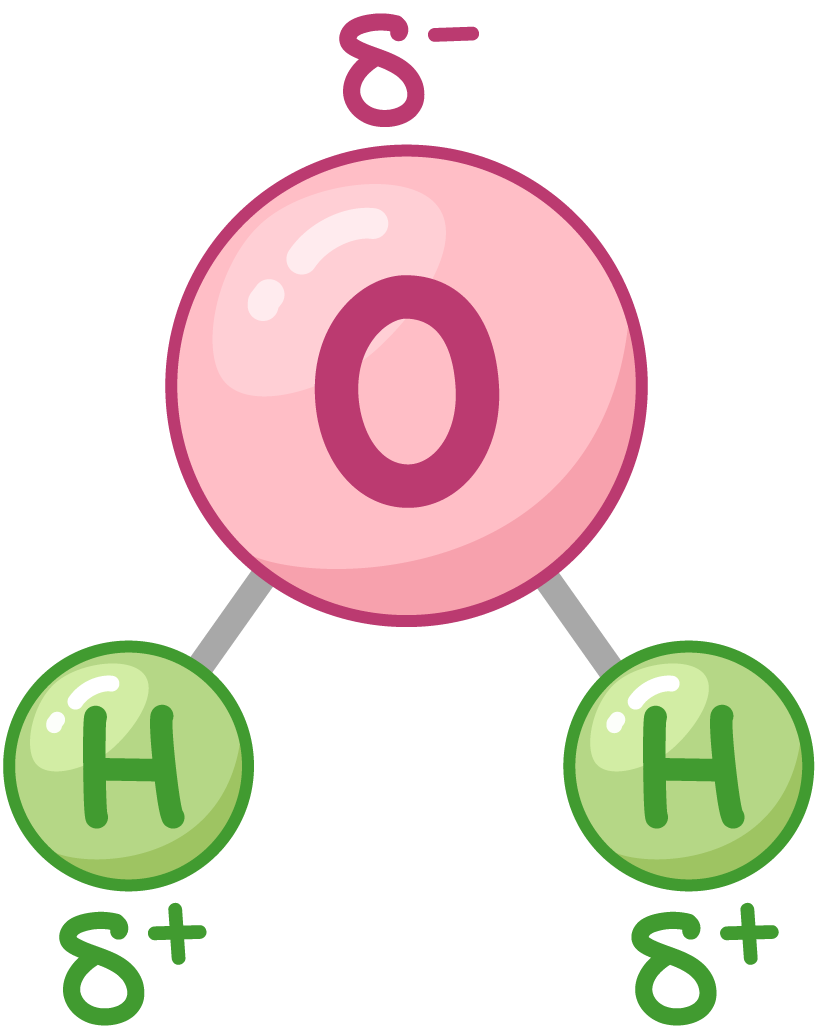 The shared electrons are pulled towards the oxygen atom, giving the oxygen atom a slightly negative charge (δ-). This leaves the hydrogen atoms with a slightly positive charge (δ+). This means that water has both positive and negative poles, making it a dipolar molecule. |
Water molecules can form hydrogen bonds Each water molecule consists of a partially negative oxygen end and a partially positive hydrogen end. This causes water molecules to interact with one another. The partially positive hydrogen end of one water molecule attracts towards the partially negative oxygen end of another molecule. This force of attraction is known as a hydrogen bond. 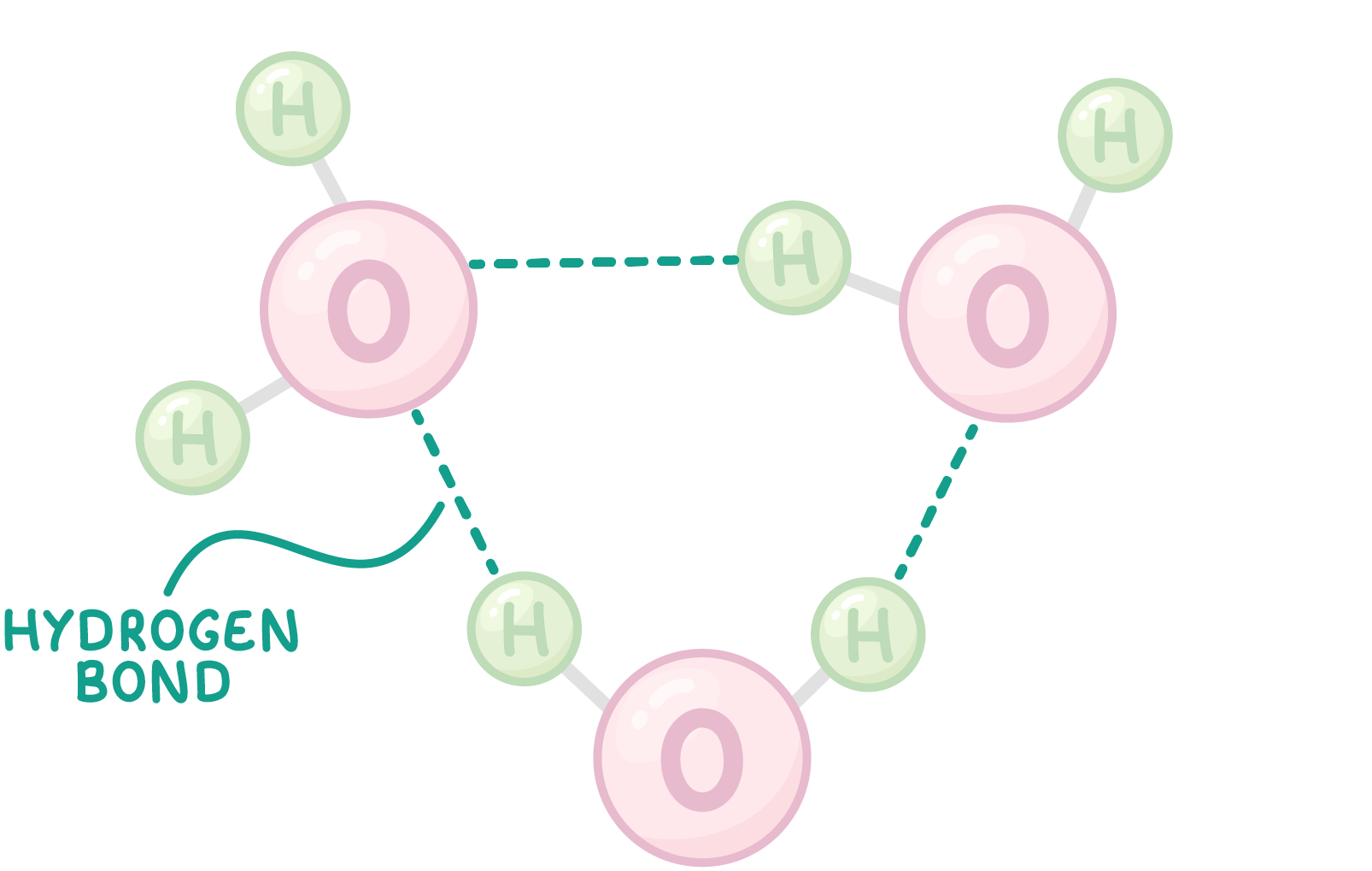 These hydrogen bonds form between many water molecules, causing them to stick together and giving water some of its useful properties. |
Roles of water Water plays many roles in living organisms. These functions include:
|
Water as a solvent Many substances within cells are ionic compounds. This means they consist of positive and negative ions (e.g. salt is made up of Na+ and Cl- ions). When these ionic compounds are added to water, the ions are split apart.  As water is polar, the slightly negative oxygens are attracted to the positive ions whilst the slightly positive hydrogens are attracted to the negative ions. Each ion is surrounded by water molecules and the compound dissolves. |
Water is known as the universal solvent because it dissolves more substances than any other liquid. This is useful for the following reasons:
|
Water as a temperature buffer Water has a high specific heat capacity. This means a lot of energy is needed to raise the temperature of 1 gram of water by 1°C. This is because the numerous hydrogen bonds between water molecules can absorb a lot of energy before being broken. This means it takes a lot of energy to break the hydrogen bonds and heat the water. The high specific heat capacity of water means that it is resistant to rapid changes in temperature. As many organisms are made up of water, this allows the body to remain at a fairly stable temperature. |
Water as a cooling mechanism Hydrogen bonding between water molecules also means that a lot of energy is needed to evaporate 1 gram of water. This means that water has a high latent heat of vaporisation (a high boiling point). A lot of energy is required to break the hydrogen bonds to change it from a liquid to a gas. This is useful for organisms because they can use evaporation of water as a method of cooling without losing too much water. When water evaporates from the surface of the skin, it takes heat energy away from the surface, cooling the organism down. |
Water as a habitat Since water has a high specific heat capacity and a high latent heat of vaporisation, it does not change temperature or evaporate easily. This provides a stable environment for many organisms to live in. However, at low temperatures, water freezes to form ice. Water molecules are held further apart in ice, making it less dense than water. This causes ice to float, forming an insulating layer at the surface of ponds and lakes. This means the water below this layer does not freeze, allowing organisms within the water to move and survive. |
Water as a metabolite Water is involved in many chemical reactions inside organisms. These reactions include:
|
Water as a transport medium The tendency of water molecules to stick together (via hydrogen bonds) is known as cohesion. Water also has a tendency to stick to other materials; this is known as adhesion. Strong cohesion and adhesion helps water to flow through organisms, carrying substances along with it. |
For example, cohesion and adhesion allow plants to transport water through the xylem in a continuous column.  |
In the same way, when water molecules meet air they create a high surface tension. This forms a skin-like structure at the surface of the water which is strong enough to support small organisms such as pond-skaters. |