ATP
This lesson covers:
- The role and structure of ATP
- The reactions which form and break down ATP
- How the properties of ATP relate to its function
- The uses of ATP in the body
What is ATP? ATP is an important molecule involved in energy transfer within cells and is often referred to as the 'energy currency' of the cell. You can think of it like a battery, temporarily holding energy and transferring it from one part of the cell to another. |
Structure of ATP ATP is a nucleotide derivative. It has a similar structure to the nucleotides (monomers) found in DNA and RNA.
ATP stands for adenosine triphosphate. 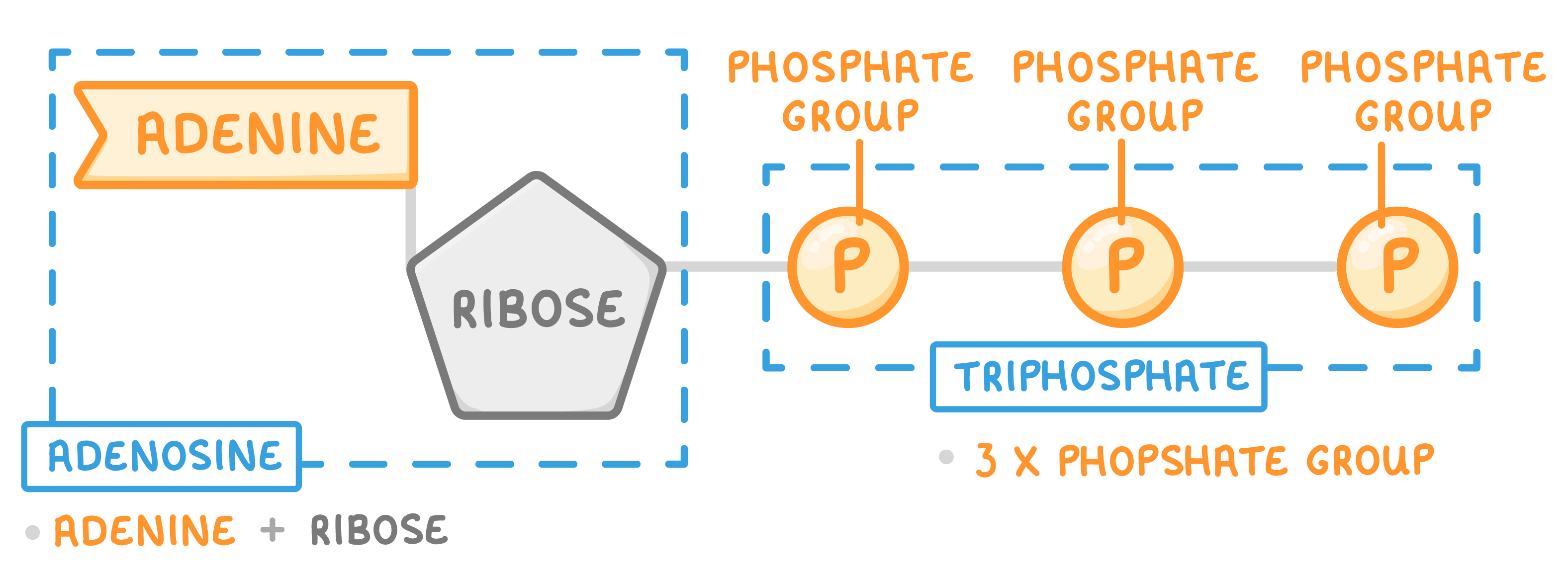 ATP consists of 3 parts:
|
The uses of ATP in the body
ATP is used for a variety of energy-requiring processes such as:
- Movement, such as muscle contraction or for sperm cells to swim.
- Active transport of molecules against the concentration gradient, such as ions entering plant roots.
- Synthesis of large molecules, such as DNA and proteins.
- Secretion of substances from cells, such as releasing hormones from glands.
ATP can also activate molecules by phosphorylating them. When ATP is hydrolysed, the phosphate can be added to other molecules (such as enzymes) to make them more reactive.
ATP reactions ATP is broken down using a hydrolysis reaction and re-synthesised using a condensation reaction:
The breakdown and re-synthesis of ATP is an example of a reversible reaction. |
Hydrolysis of ATP to release energy 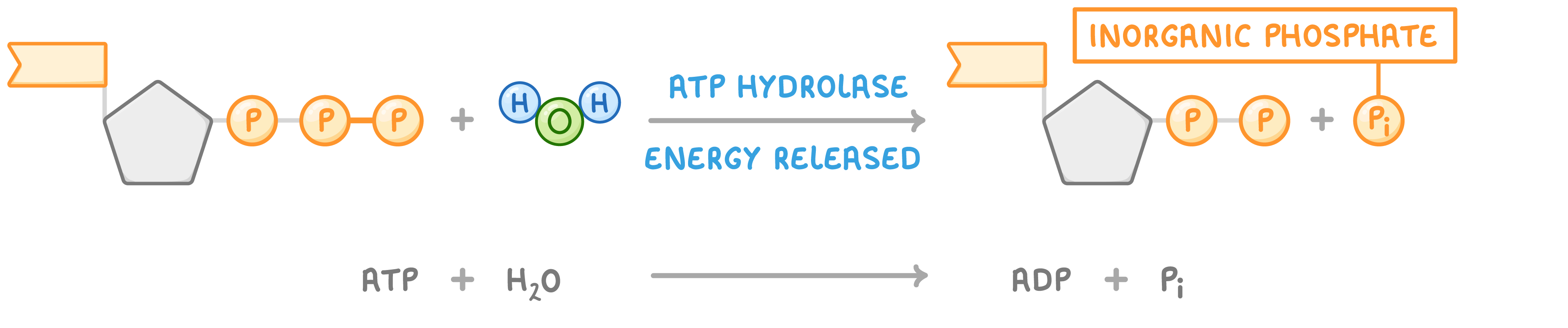 When water is added to ATP, it breaks down into adenosine diphosphate (ADP) and inorganic phosphate (Pi). This reaction is catalysed by the enzyme ATP hydrolase and releases energy for use in cells. |
Condensation reaction to reform ATP 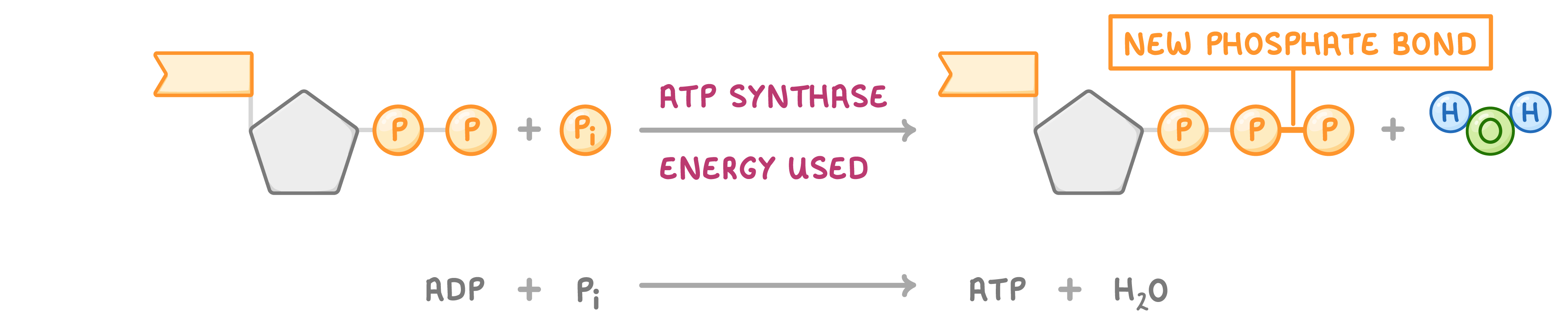 When a phosphate group and ADP join, a water molecule is released. This reaction is catalysed by the enzyme ATP synthase. This process requires energy and traps chemical energy in the bond. |
How the properties of ATP relate to its function
ATP is an immediate energy source and does not work well as a long-term energy store.
The following features allow ATP to work well as an immediate energy source:
- The hydrolysis of ATP releases a small amount of energy, meaning little energy is lost as heat.
- ATP is broken down in one step, meaning energy is released quickly.
- ATP is rapidly re-synthesised so that ATP is always readily available.
- The inorganic phosphate from ATP hydrolysis can phosphorylate other compounds, which makes them more reactive.
- The bonds between the phosphate groups are unstable, have a low activation energy and are easily broken.
- ATP is soluble, so it can easily be transported around cells.