Lipids: Introduction
This lesson covers:
- The roles of lipids in living organisms
- The difference between saturated and unsaturated fatty acids
- How to test for lipids
What are lipids? Lipids are biological molecules that contain the elements carbon (C), hydrogen (H), and oxygen (O). However, lipids contain a much lower proportion of oxygen than carbohydrates. Lipids are not made up of long chains of monomers, meaning they are not considered as polymers. |
Roles of lipids The main functions of lipids:
|
Fatty acids Most lipids are made up of fatty acids combined with an alcohol (usually glycerol). 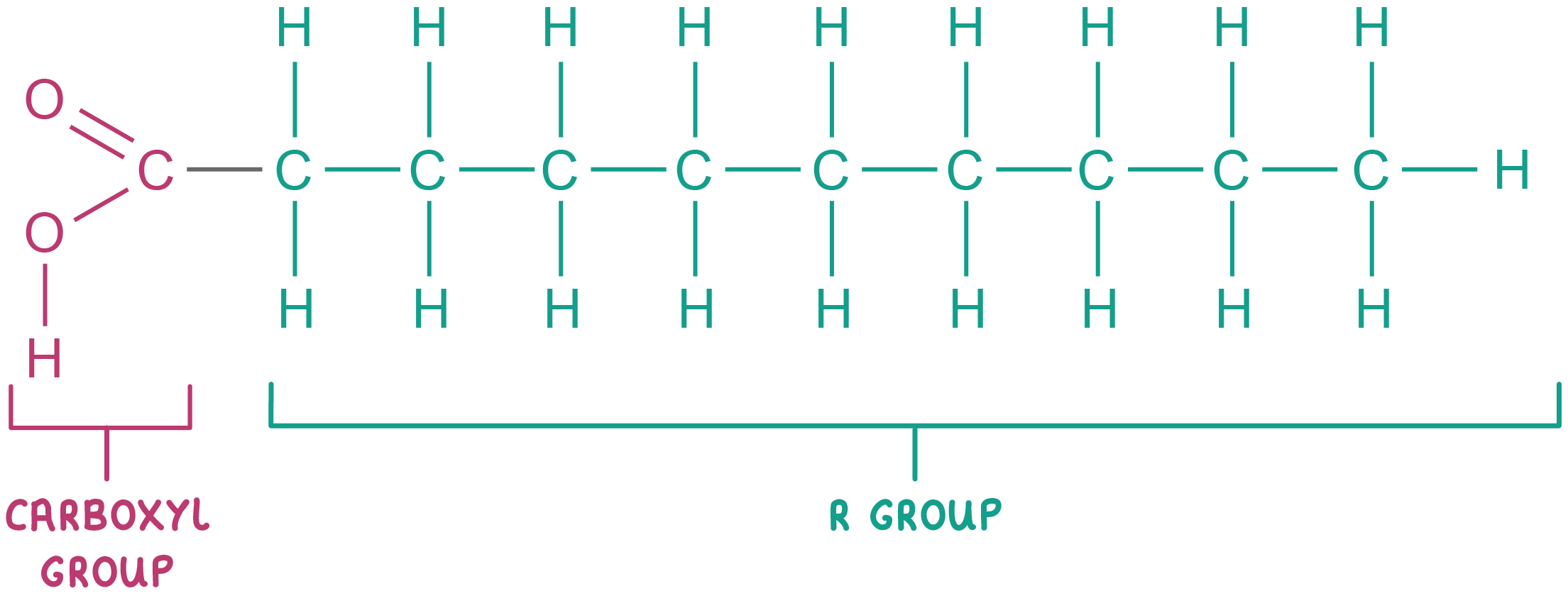 Fatty acids consist of a carboxyl group (-COOH) attached to a hydrocarbon chain (R group). |
Saturated fatty acids and unsaturated fatty acids There are two types of fatty acid: saturated fatty acids and unsaturated fatty acids. Saturated fatty acids:
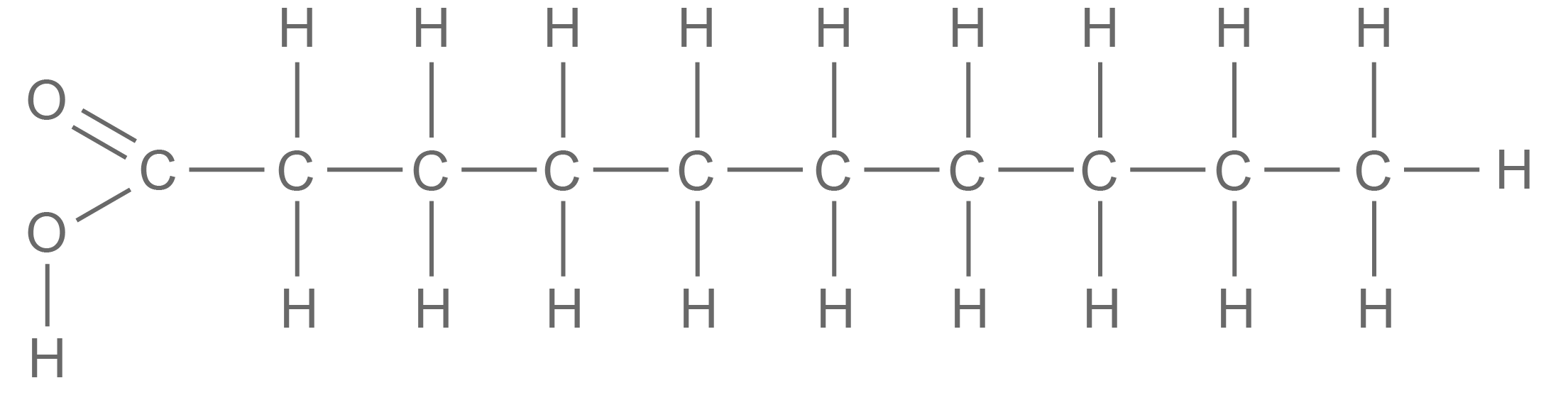 |
Unsaturated fatty acids:
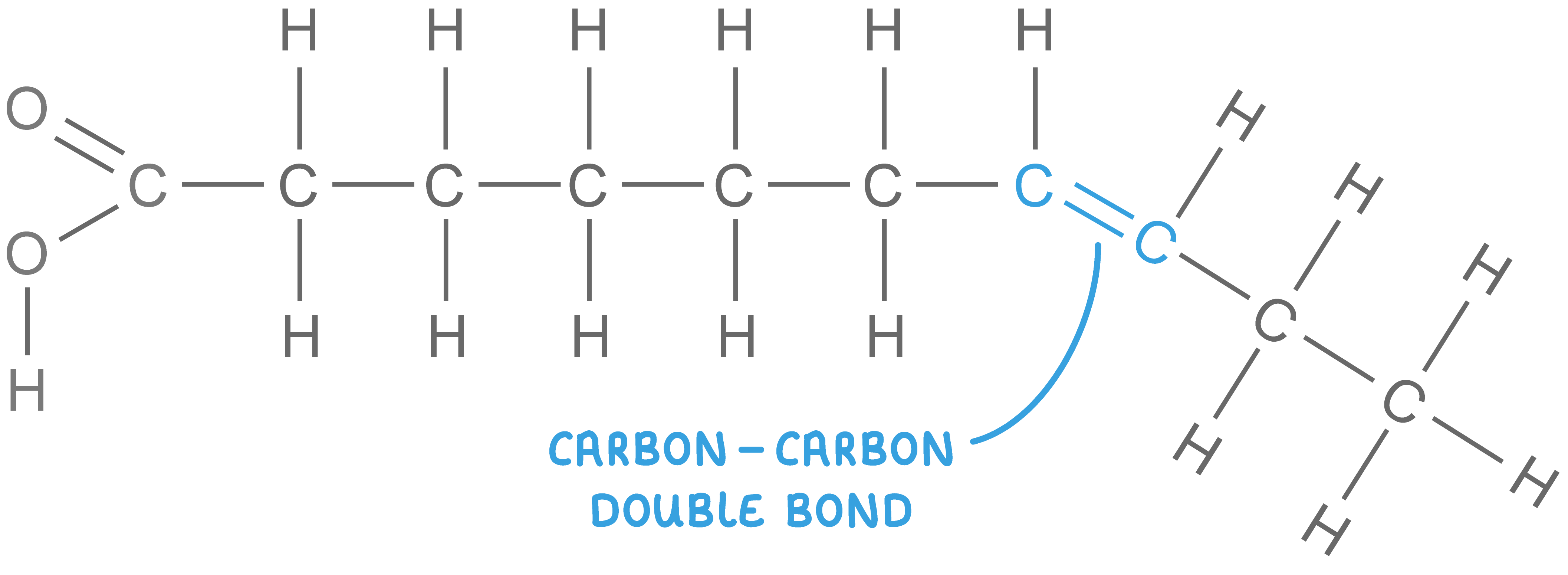 Unsaturated fatty acids may either be monounsaturated or polyunsaturated:
|
Testing for lipids
To find out whether a sample contains lipids, you must carry out the emulsion test.
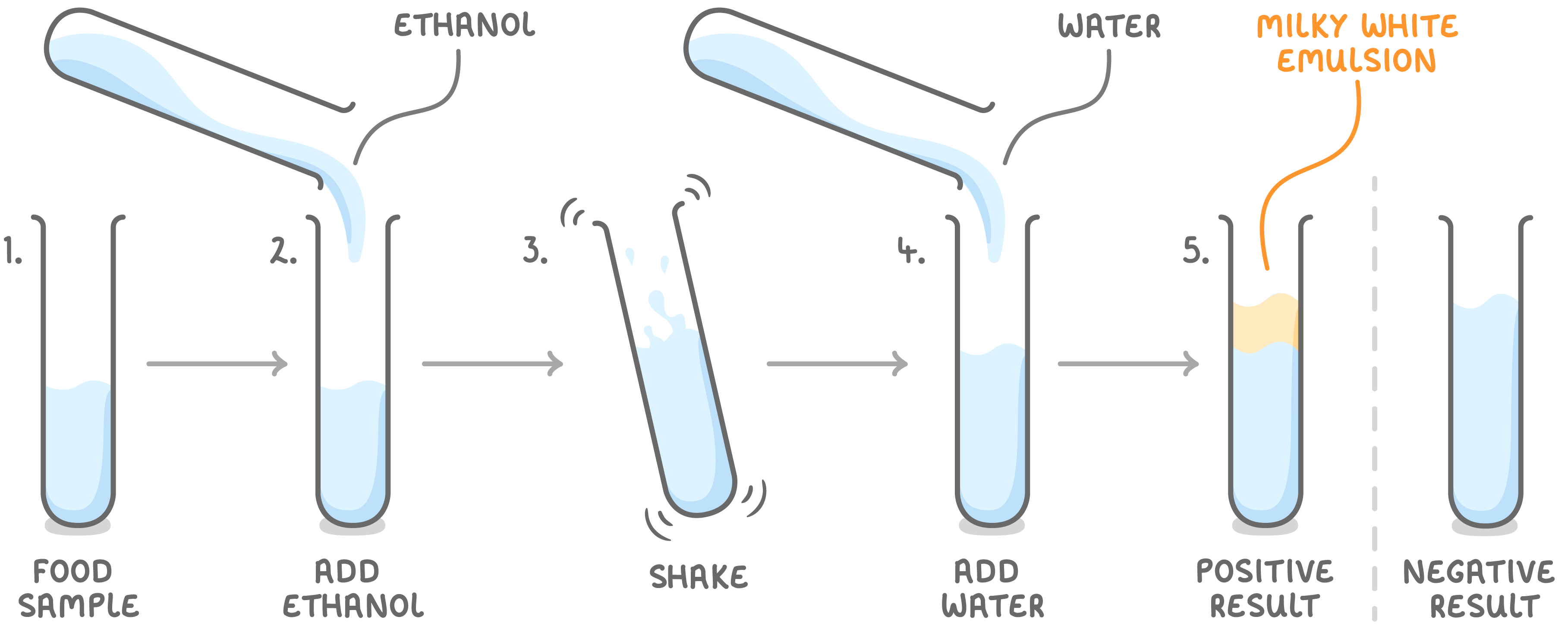
Steps to find out whether a sample contains lipids:
- Place your food sample in a test tube.
- Add 2 cm3 of ethanol.
- Shake.
- Add 2 cm3 of distilled water.
- If lipids are present, a milky white emulsion will appear.