Osmosis
This lesson covers:
- The definition of water potential
- The process of osmosis
- Factors affecting the rate of osmosis
Solutions and water potential In order to understand osmosis, we first need to understand what solutions are and what the term water potential means. Solutions are mixtures made up of a solute (e.g. glucose), dissolved in a solvent (e.g. water). Water potential (Ψ) is the pressure exerted by water molecules on the membrane (or container) surrounding a solution. It is measured in kiloPascals (kPa). High and low water potential:
|
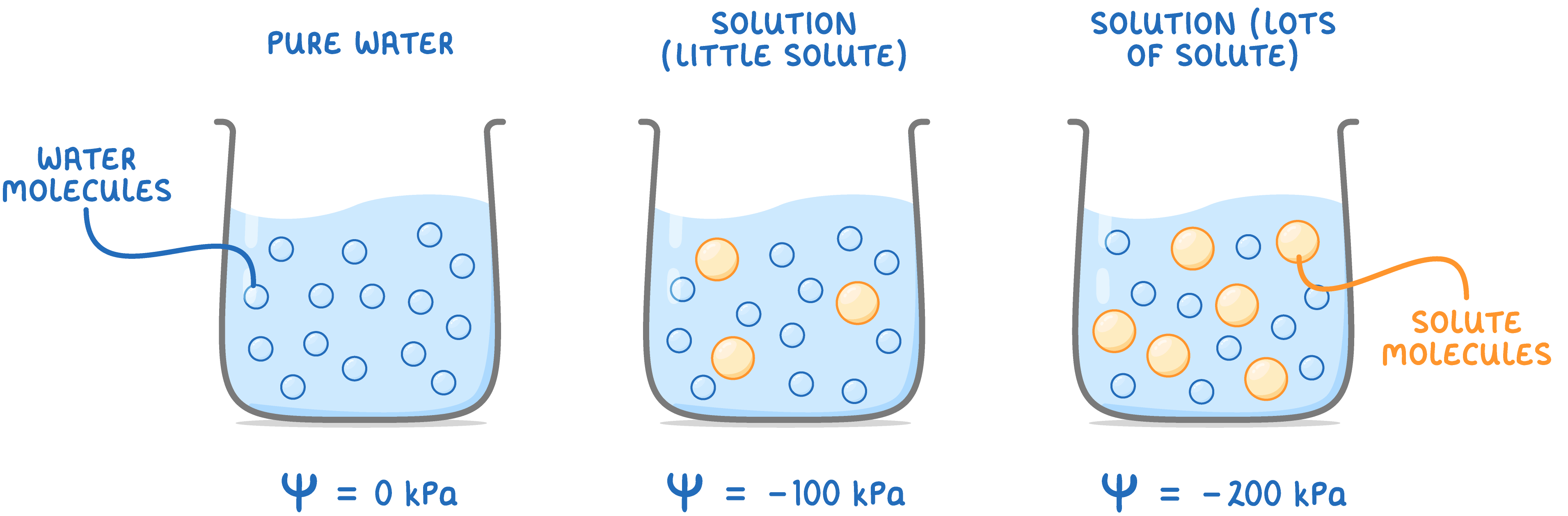 |
Pure water has a water potential of 0 kPa, and the value decreases (becomes more negative) as more solute is added. |
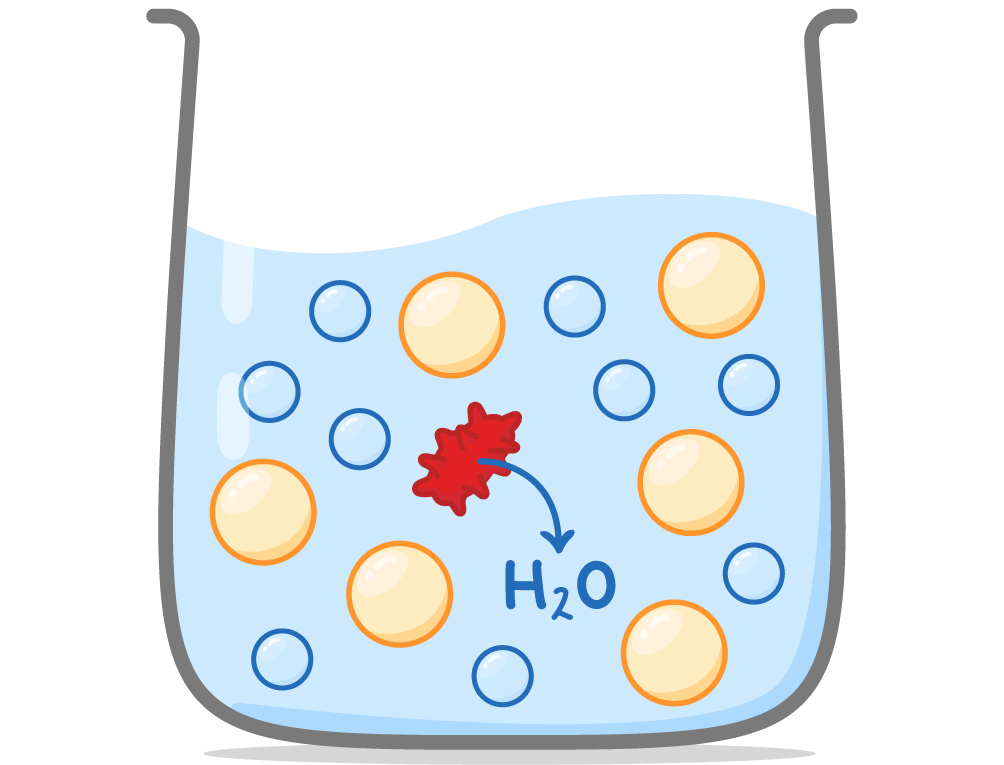
Osmosis Osmosis is the diffusion of water molecules across a partially permeable membrane from an area of higher water potential to an area of lower water potential. 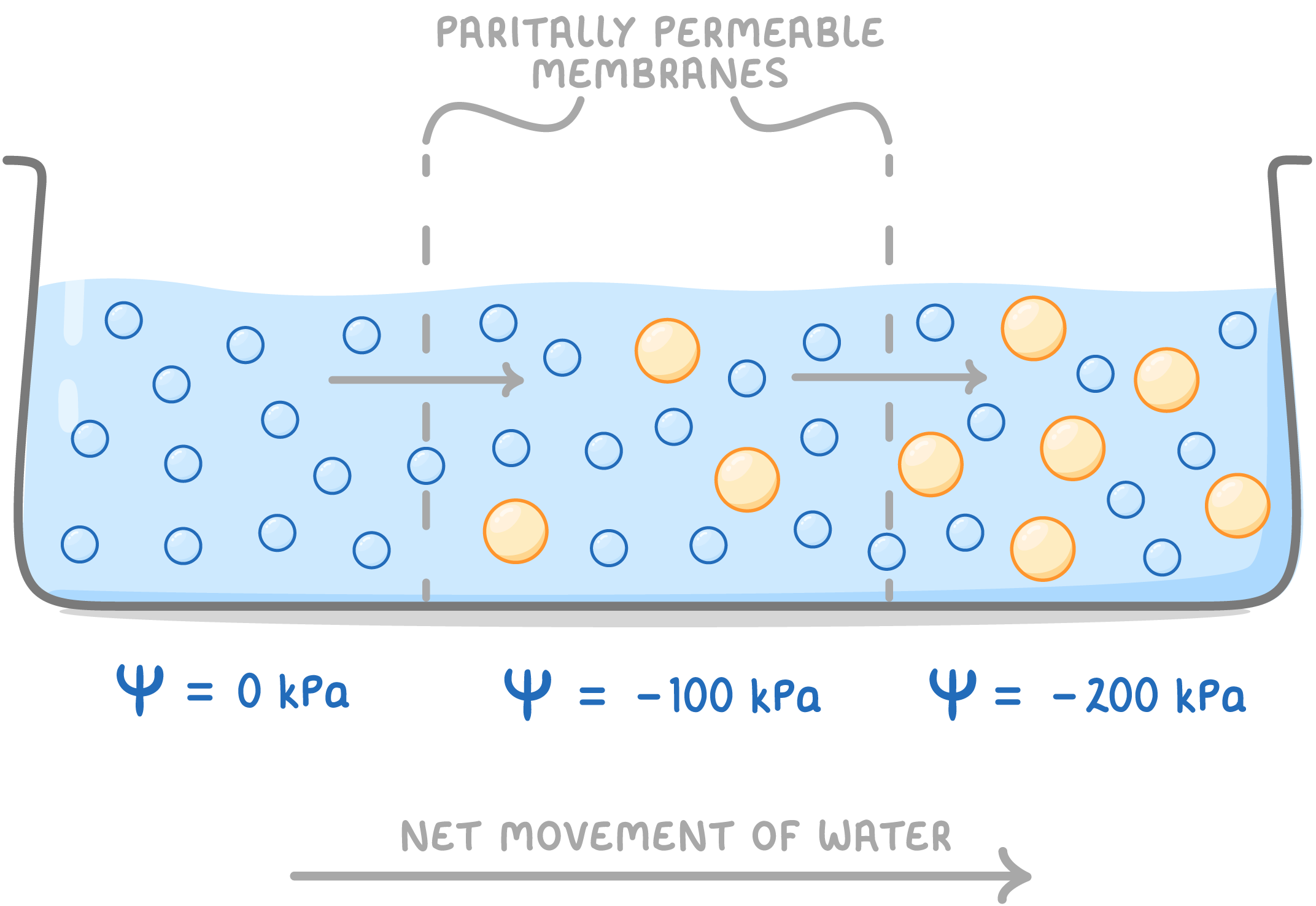 |
Water molecules are small and can diffuse directly through the cell membrane, whereas large solute molecules cannot. Water molecules diffuse down a water potential gradient until the water potential is equal on both sides of the membrane (equilibrium). |
Osmosis in animal cells When animal cells are placed in solutions, water may move into or out of the cells depending on the water potential of the solution.  |
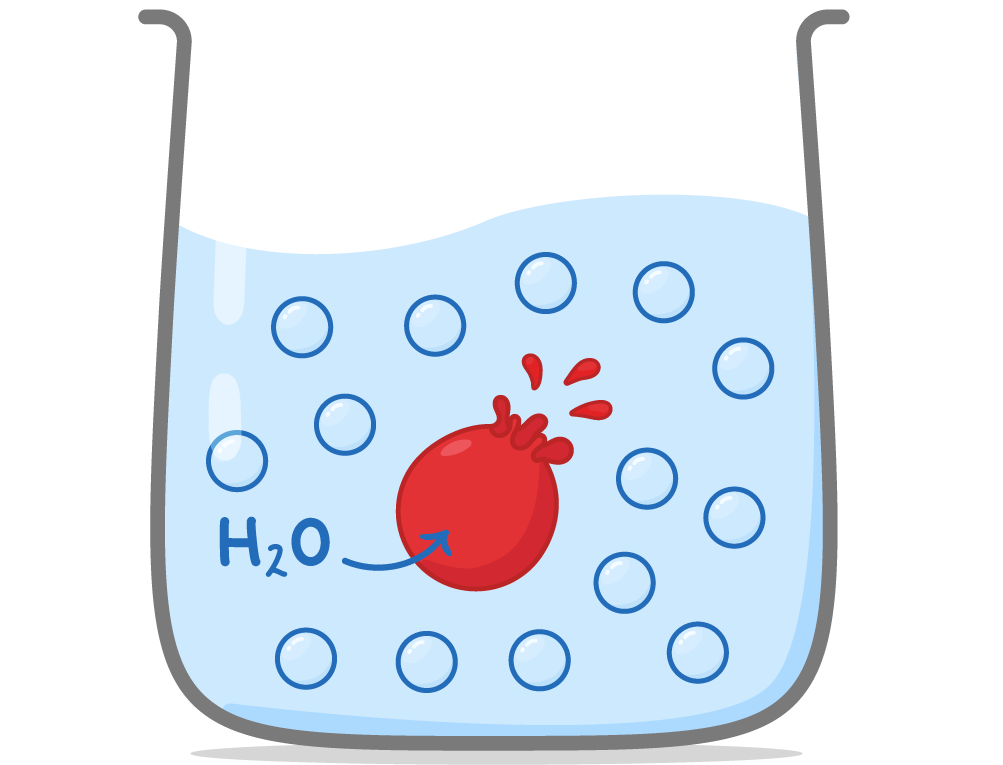 Hypotonic solutions:
|
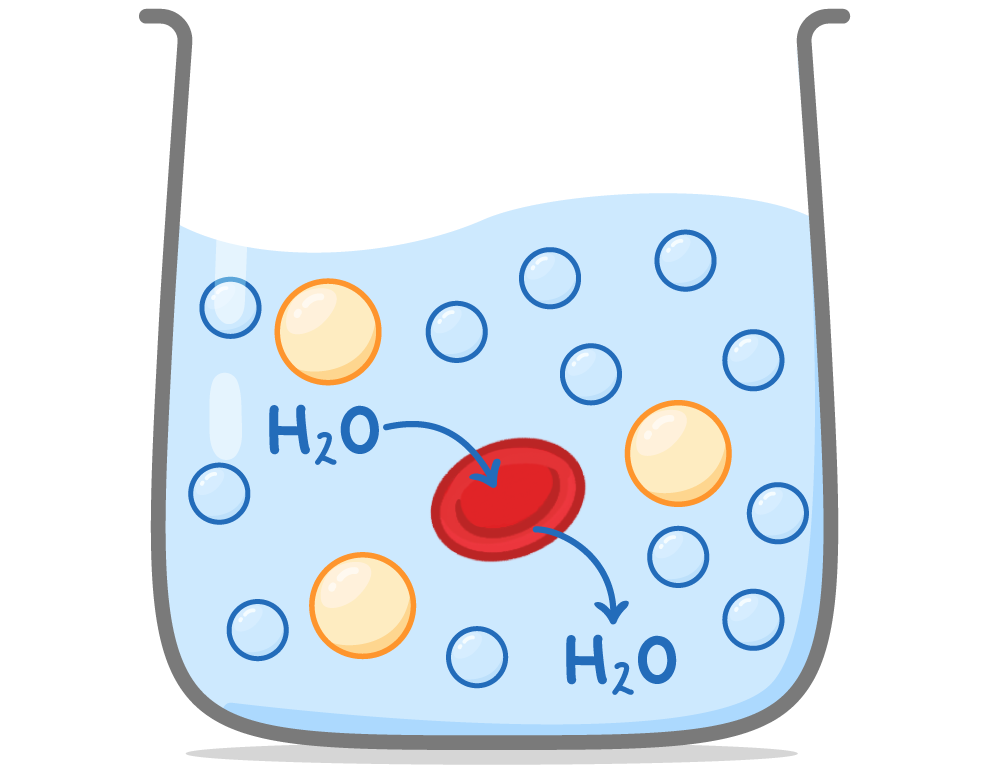 Isotonic solutions:
|
 Hypertonic solutions:
|
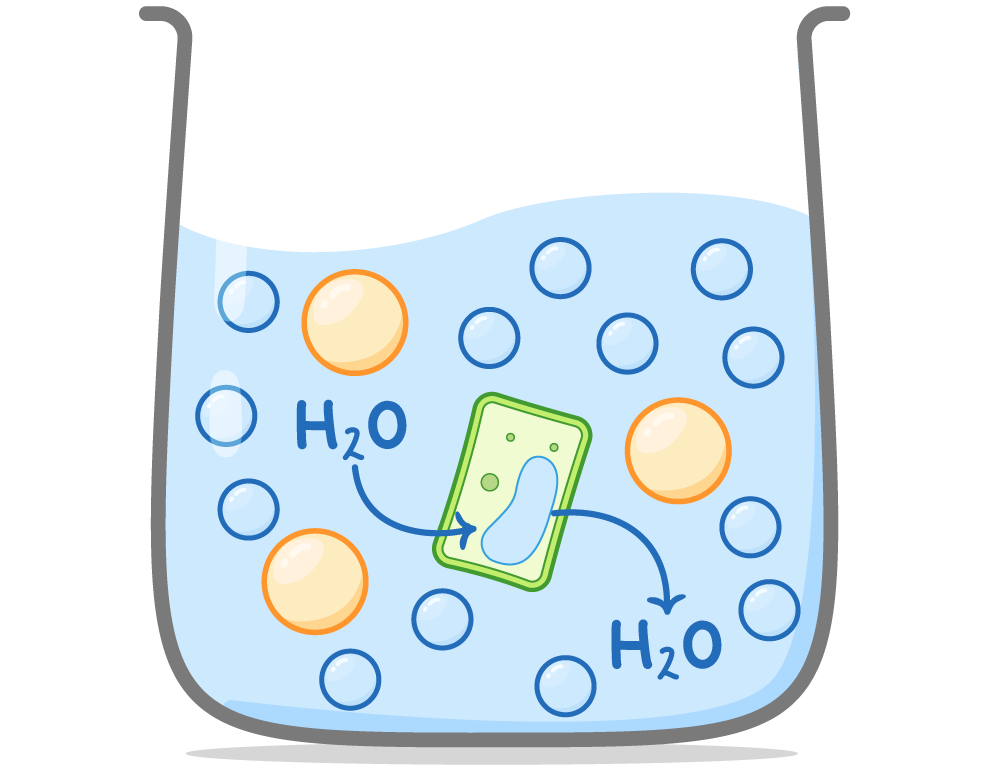
Osmosis in plant cells Unlike animal cells, plant cells do not burst due to the presence of a cell wall. 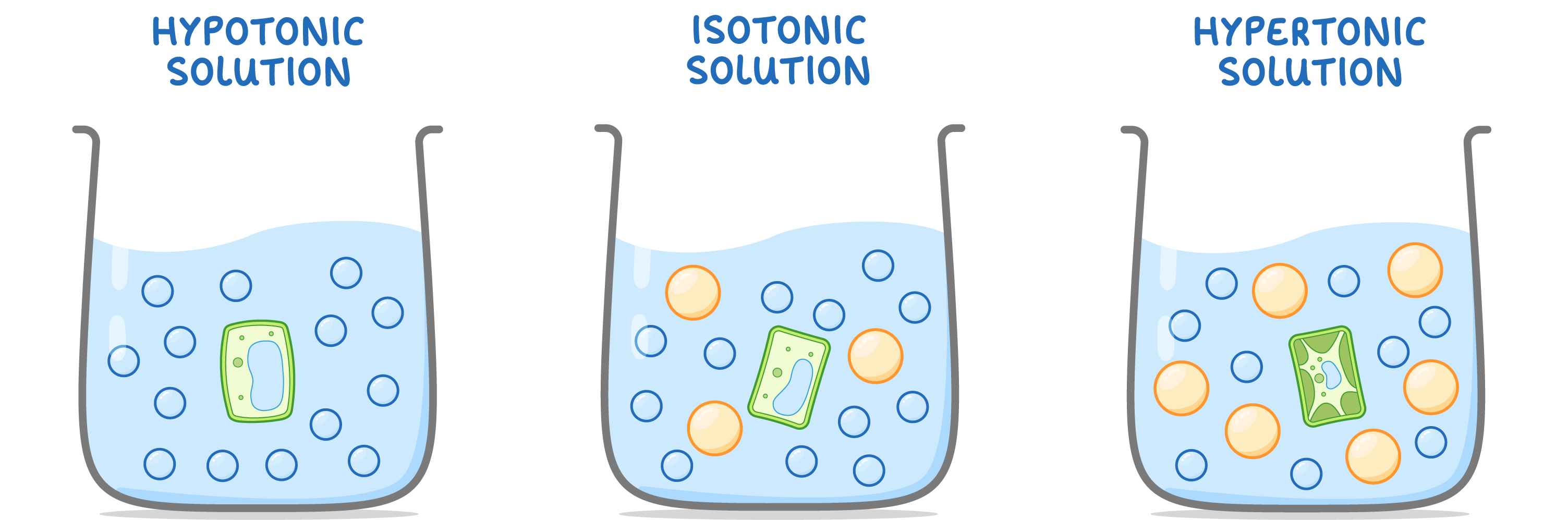 |
 Hypotonic solutions:
|
 Isotonic solutions:
|
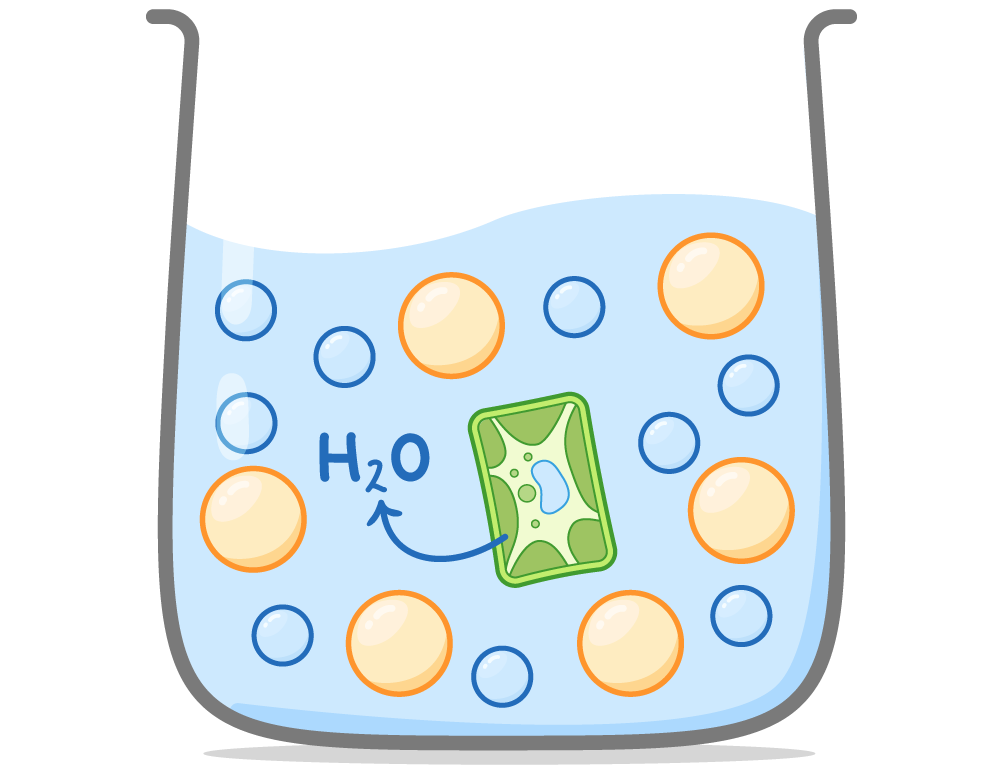 Hypertonic solutions:
|
Factors affecting the rate of osmosis There are four key factors that affect the rate of osmosis:
|