Lipids: Triglycerides & Phospholipids
This lesson covers:
- The structure and function of triglycerides
- The synthesis and breakdown of triglycerides
- The structure and function of phospholipids
- The similarities and differences between triglycerides and phospholipids
Triglycerides A triglyceride is a type of lipid used as a store of energy in animals, plants, and some bacteria. 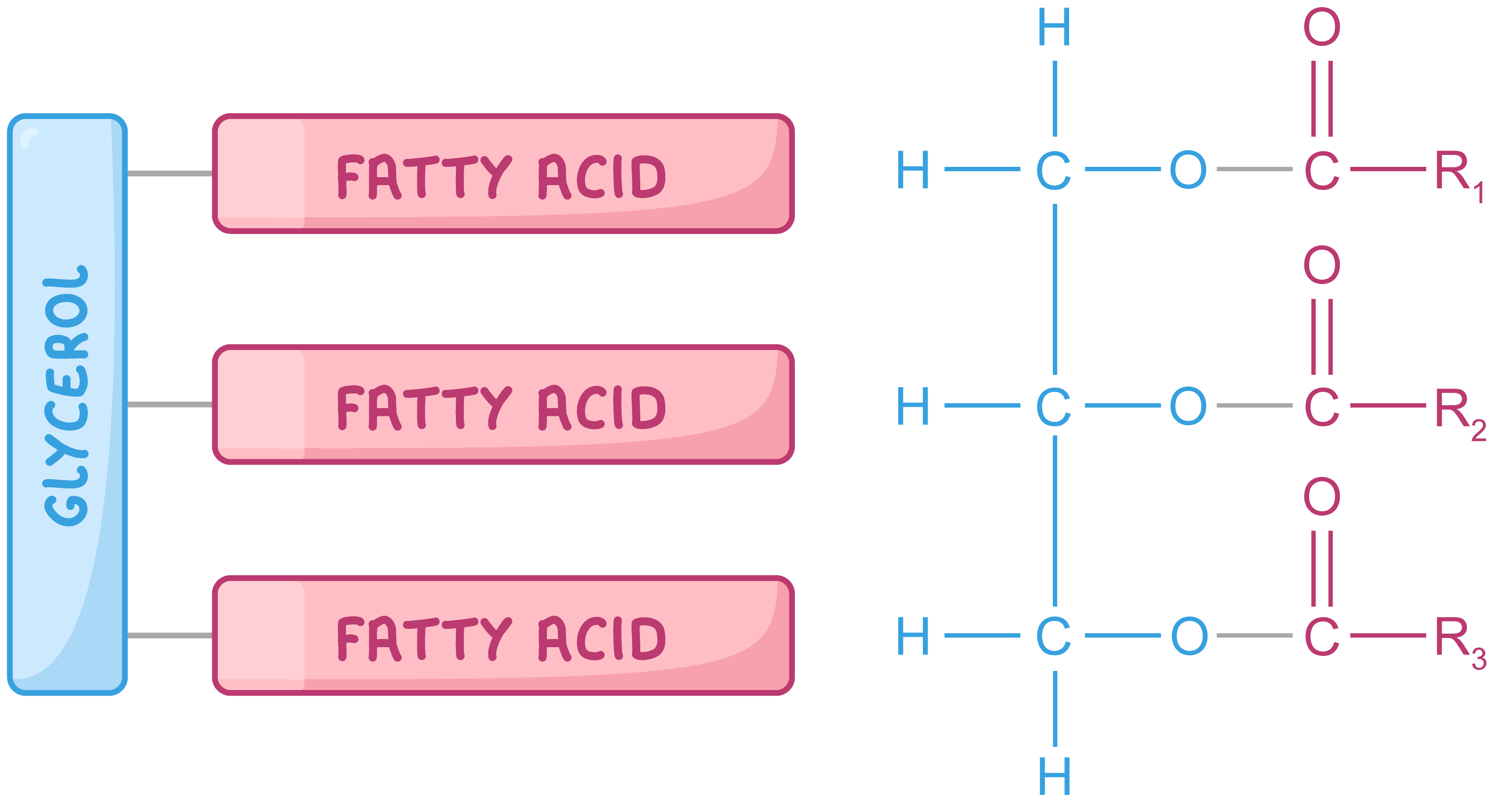 The left diagram is a simplified version of a triglyceride. The diagram on the right shows the atoms a triglyceride is made up of. A triglyceride consists of a glycerol backbone attached to three fatty acid tails. Each fatty acid tail contains a hydrocarbon chain (R) which can vary in length and may be saturated or unsaturated. |
Features that allow triglycerides to store energy efficiently:
|
Triglyceride formation and breakdown Triglycerides are synthesised via condensation reactions and broken down via hydrolysis reactions. These reactions involve the formation or the breakdown of covalent bonds known as ester bonds. |
Condensation reaction 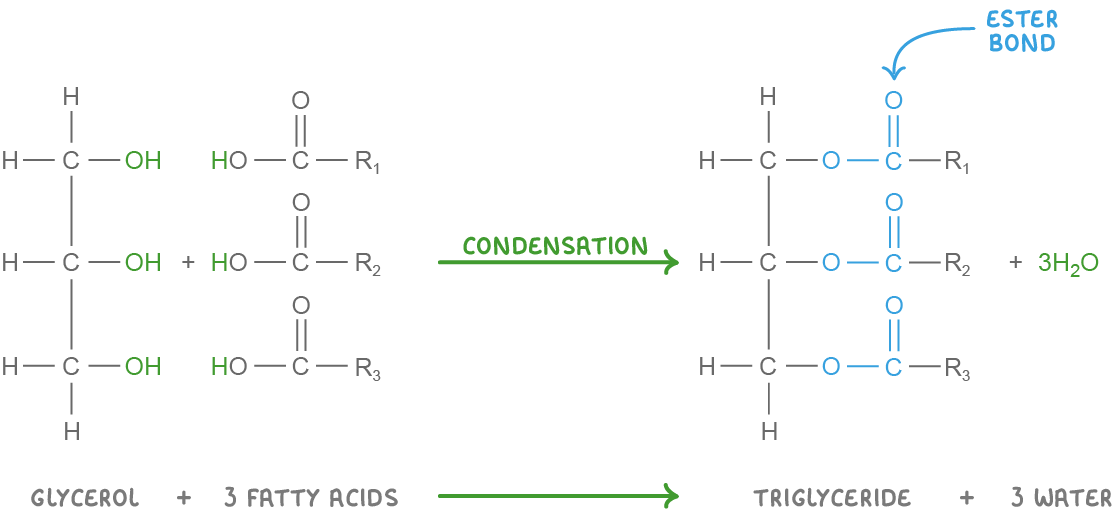 Condensation:
|
Hydrolysis reaction 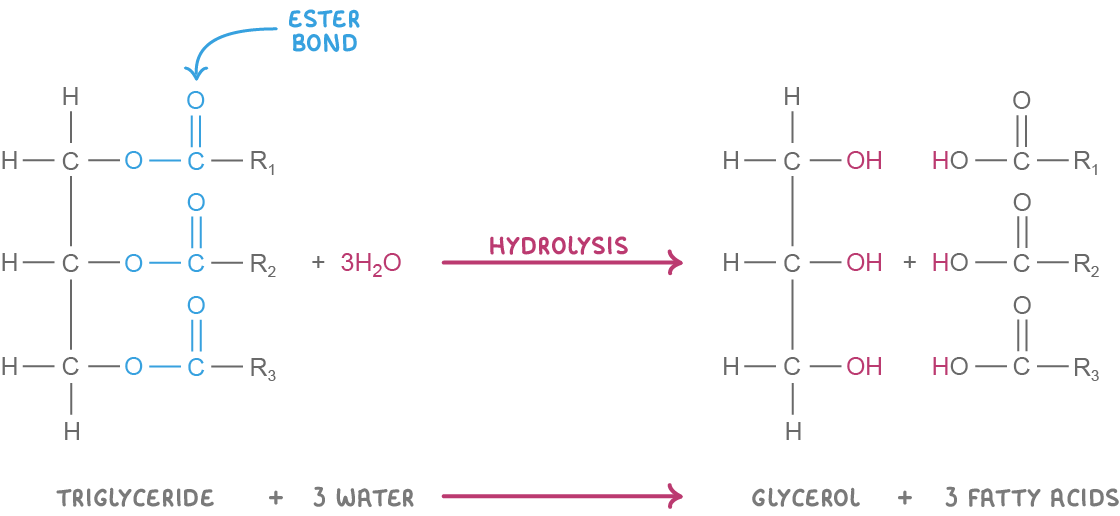 Hydrolysis:
|
Phospholipids A phospholipid is a type of lipid used as a structural component of the cell membrane. 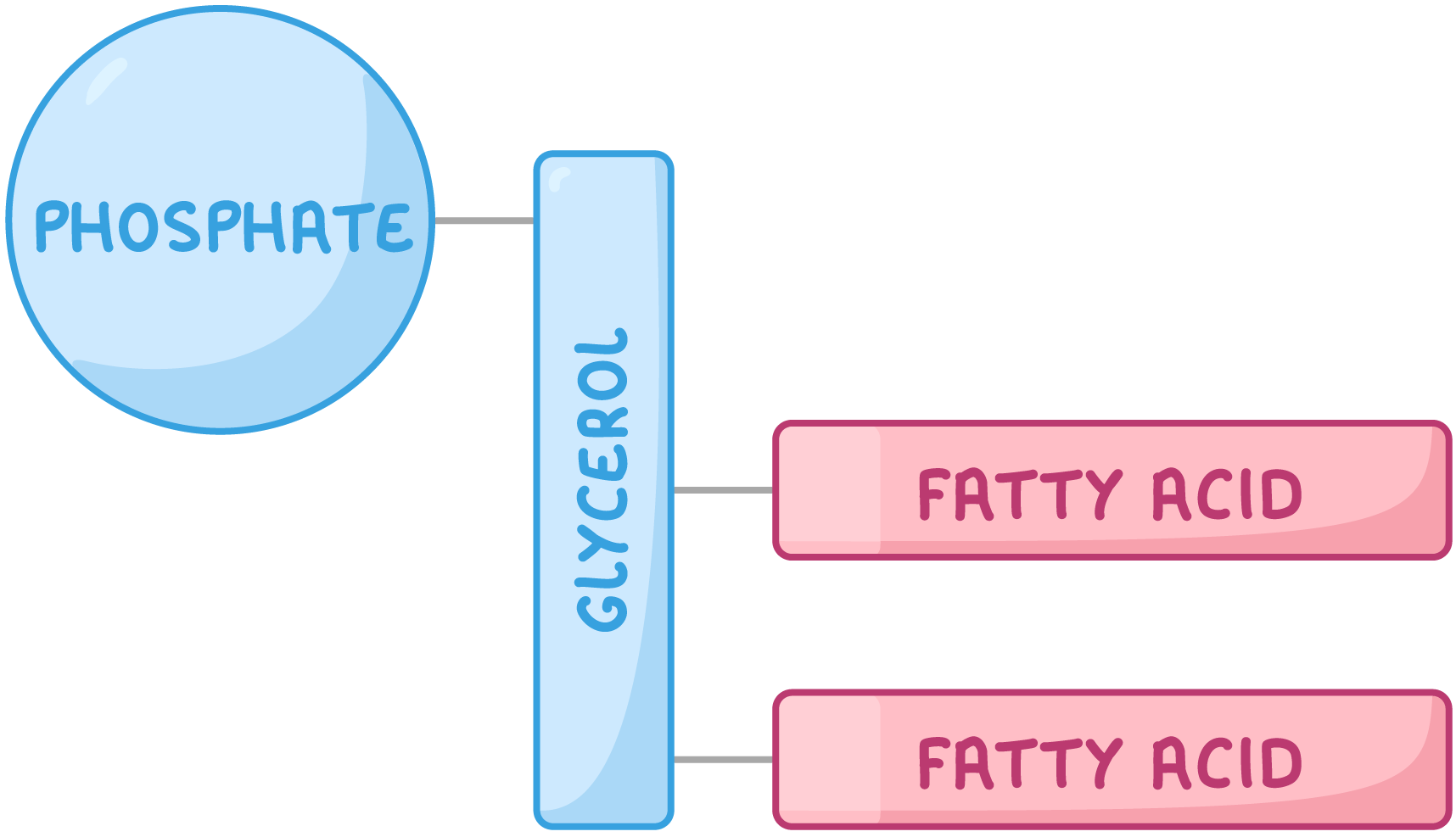 They are similar to triglycerides except one of the fatty acid tails is replaced by a phosphate group. |
Phospholipids are polar A phospholipid is made up of two parts:
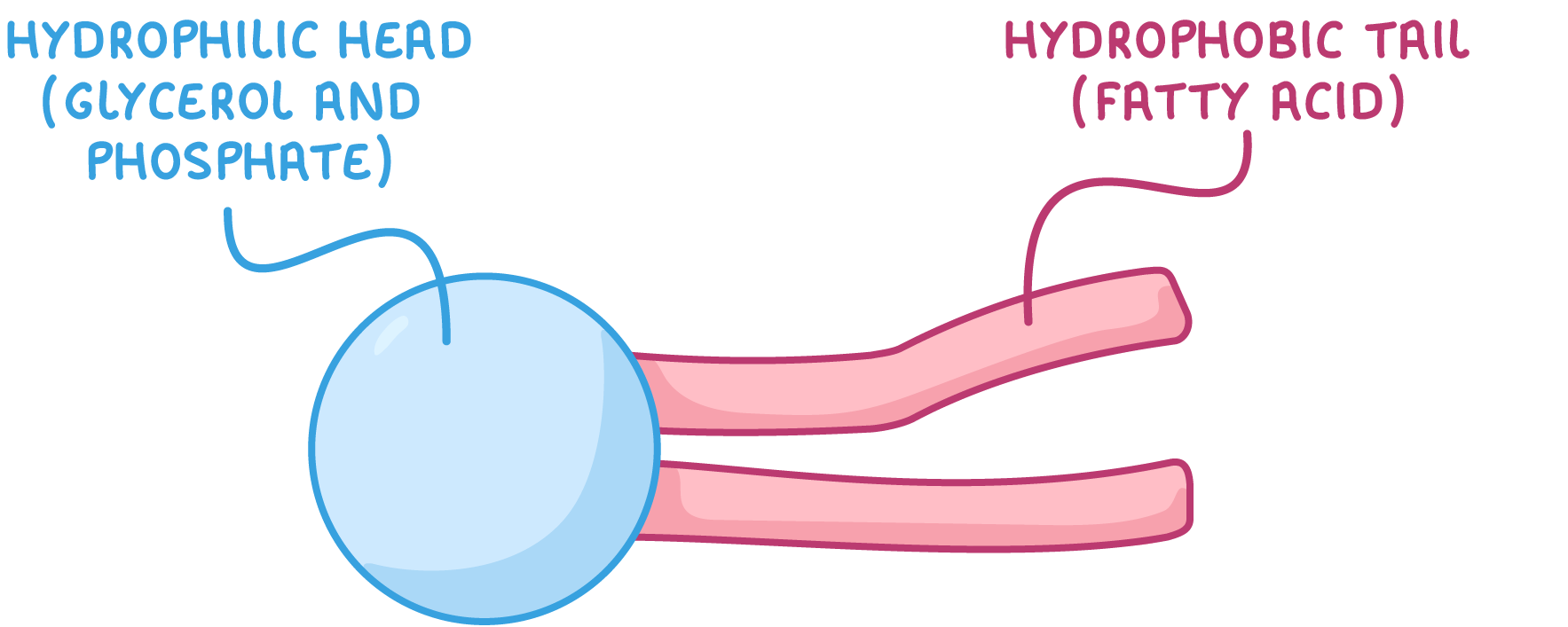 The phosphate group is polar and so attracts water (hydrophilic) whereas the fatty acid tails repel water (hydrophobic). |
Phospholipid bilayer When phospholipids are placed in water, they arrange themselves into a double layer (bilayer) so that the hydrophilic heads are facing out (towards the water) and the hydrophobic tails are facing in (away from the water). 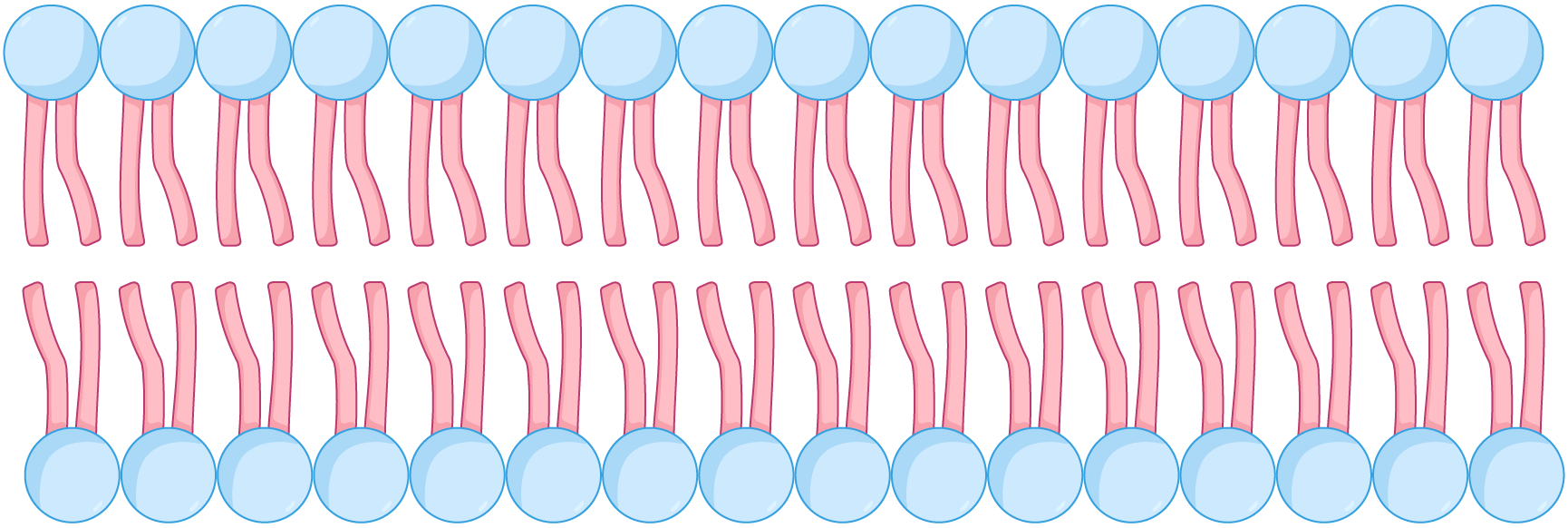 This arrangement creates a hydrophobic centre in the bilayer so that water-soluble substances cannot pass through. |
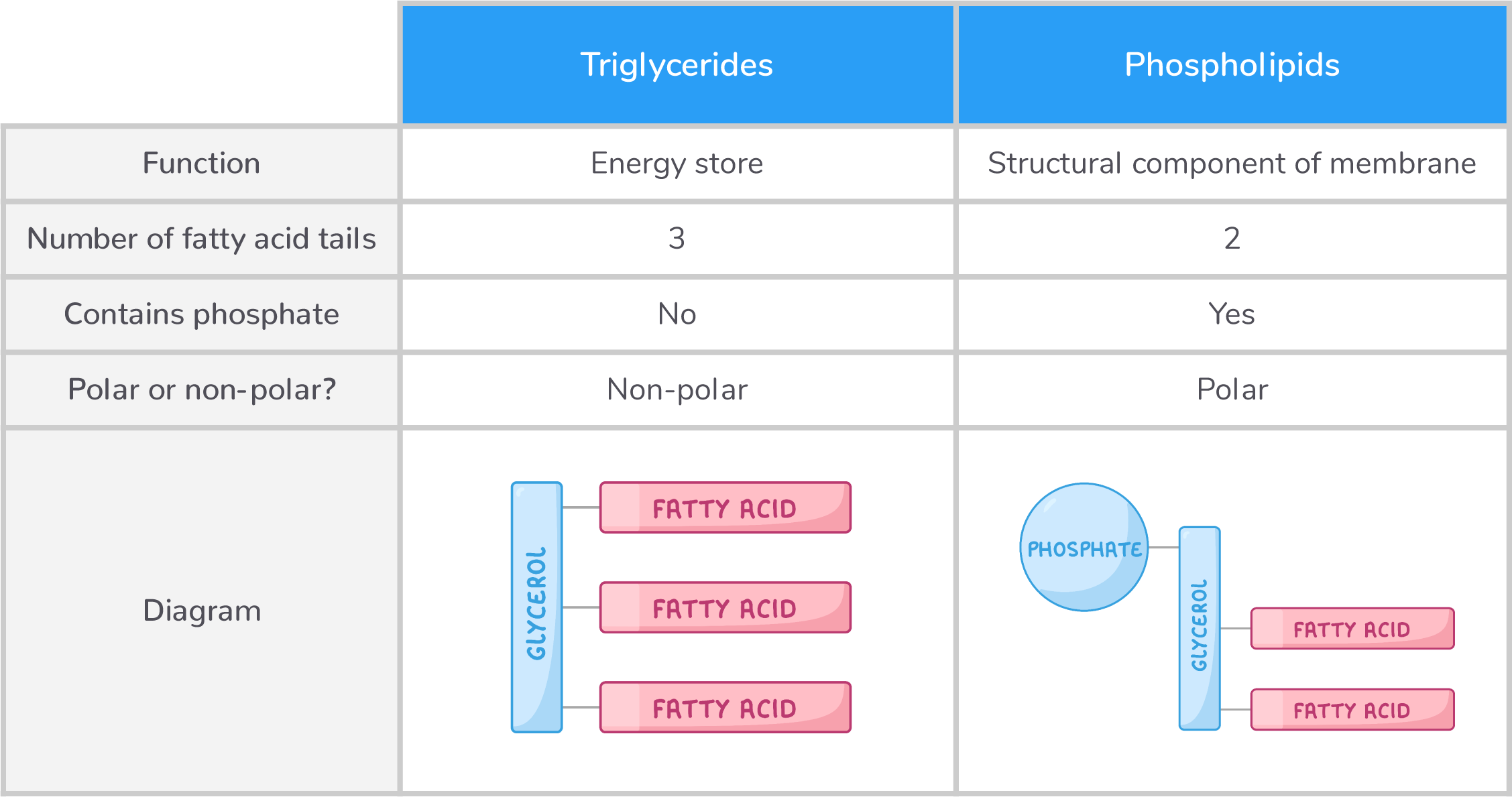
Comparing triglycerides and phospholipids