Carbohydrate: Polysaccharides
This lesson covers:
- The different types of polysaccharides: starch, glycogen, and cellulose
- How their structures relate to their functions
Polysaccharides
Polysaccharides are complex carbohydrates made up of many monosaccharides joined via glycosidic bonds.
Examples of polysaccharides include starch, glycogen, and cellulose.
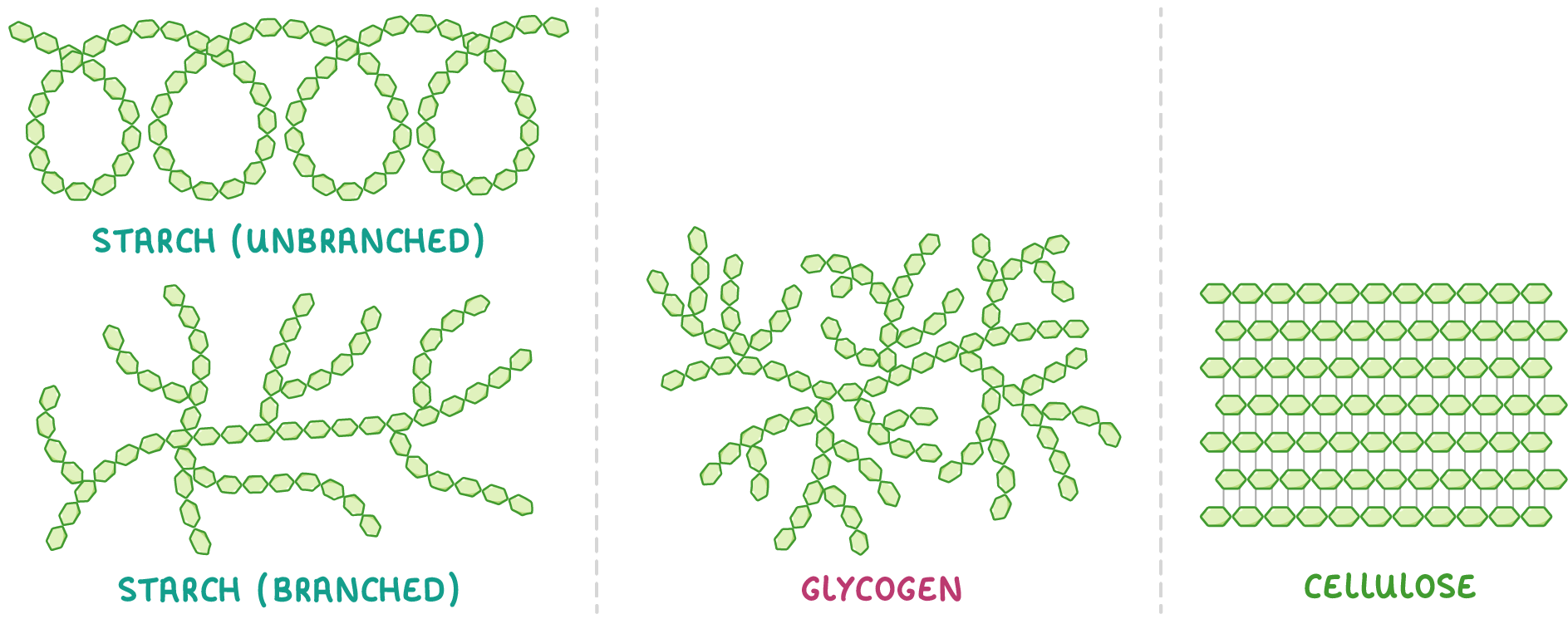
Starch Starch is an example of a polysaccharide used by plants to store excess glucose. This means that starch can be hydrolysed back into glucose when plants require energy. 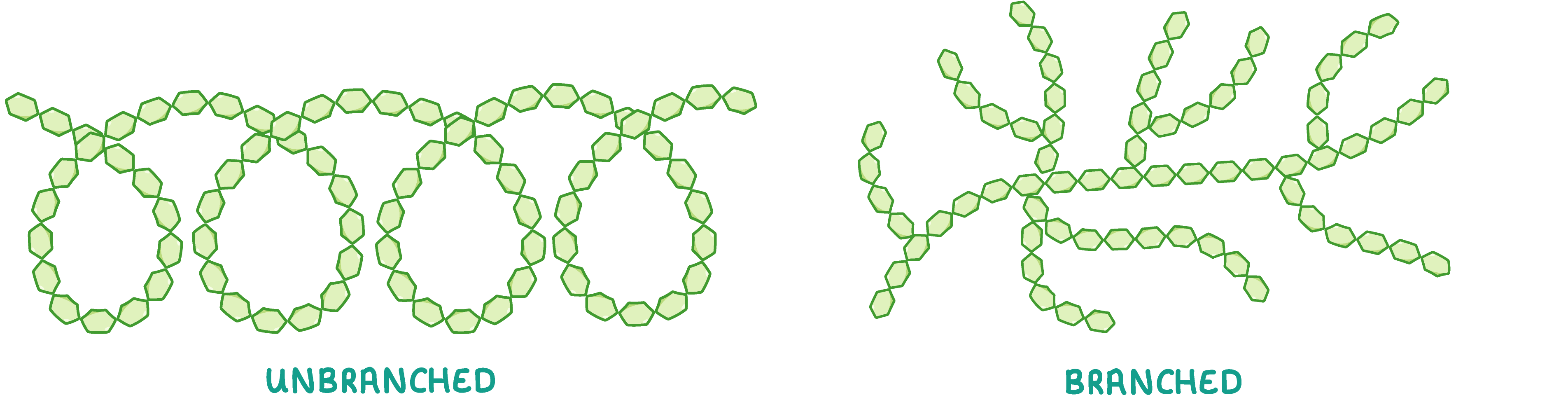 Starch is made up of many alpha-glucose monomers joined via 1-4 and 1-6 glycosidic bonds to form chains. These chains come in two forms: unbranched and branched. |
The following features allow starch to work well as a store of energy:
|
Glycogen Glycogen is an example of a polysaccharide used by animals to store excess glucose. This means that glycogen can be hydrolysed back into glucose when animals require energy. Glycogen is very similar to starch, but it is used by animals rather than plants. 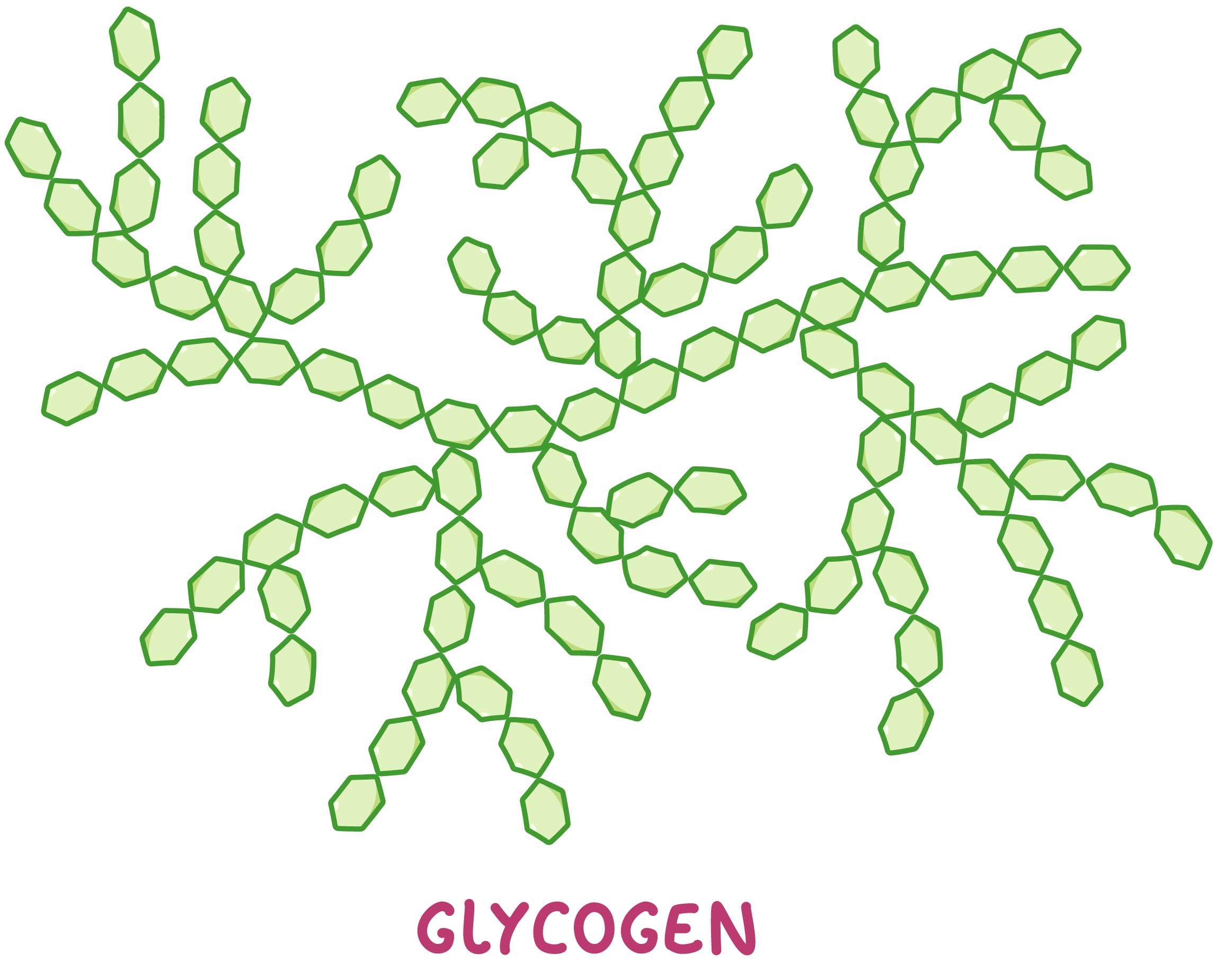 Glycogen is made up of many alpha-glucose monomers joined via 1-4 and 1-6 glycosidic bonds to form highly branched chains. |
The following features allow glycogen to function as a store of energy:
|
Cellulose Cellulose is a polysaccharide formed from beta-glucose. Its primary use is to provide structural support for plant cell walls. |
Every other beta-glucose molecule must flip upside down  Cellulose is made up of many beta-glucose monomers joined together via glycosidic bonds. However, if two beta-glucose monomers line up next to each other, the hydroxyl groups on carbon 1 and carbon 4 are too far from each other to react. |
To fix this, every other beta-glucose molecule is inverted by 180° (flipped upside down). This brings the hydroxyl groups (OH) close enough together to react. 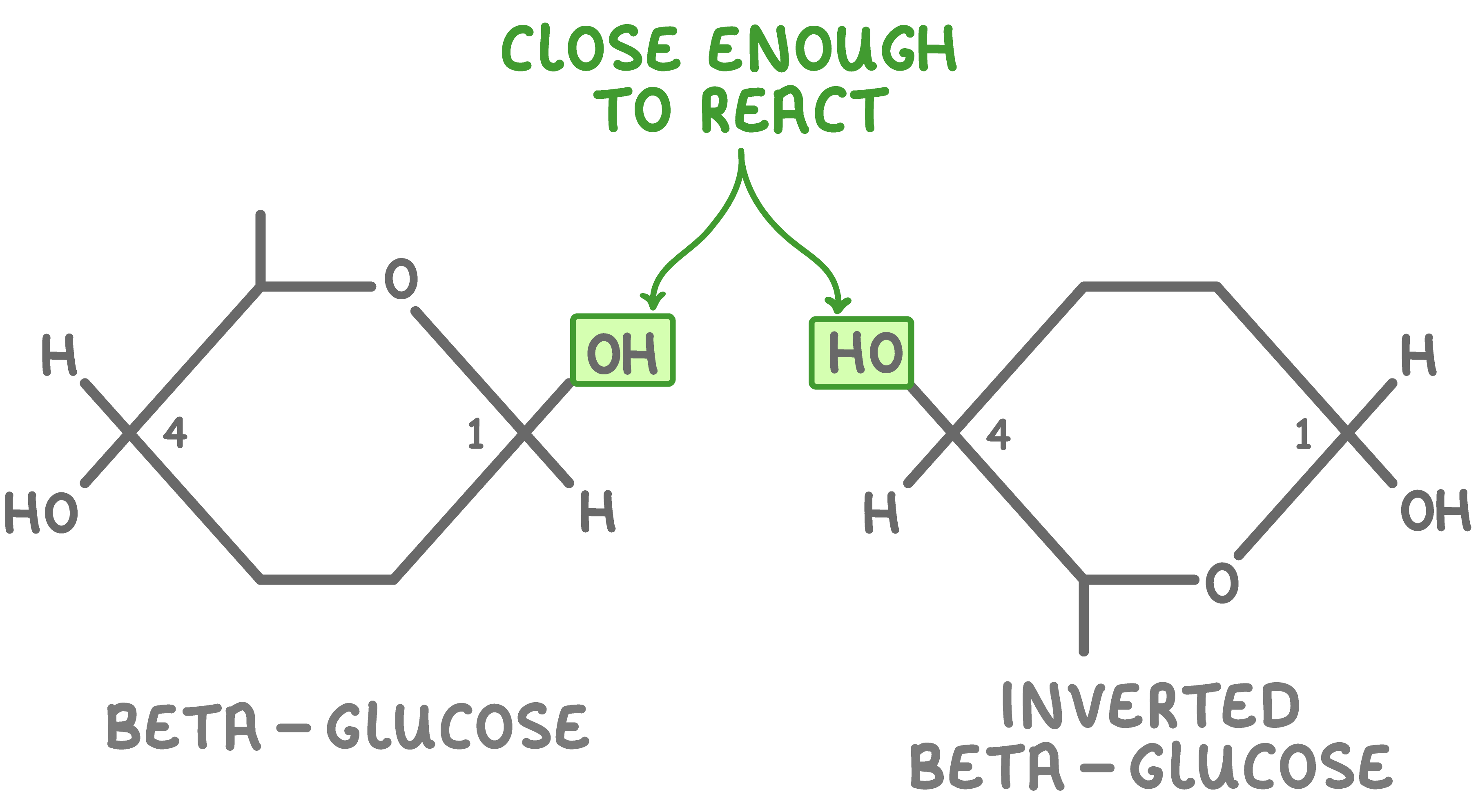 |
Many beta-glucose form long straight chains When many beta-glucose monomers join together they form long, straight, unbranched chains. The alternating inversion of the beta glucose molecules also allows for hydrogen bonds to form between individual chains. Although each hydrogen bond itself is relatively weak, the huge number of these bonds provides great strength to cellulose as a whole. 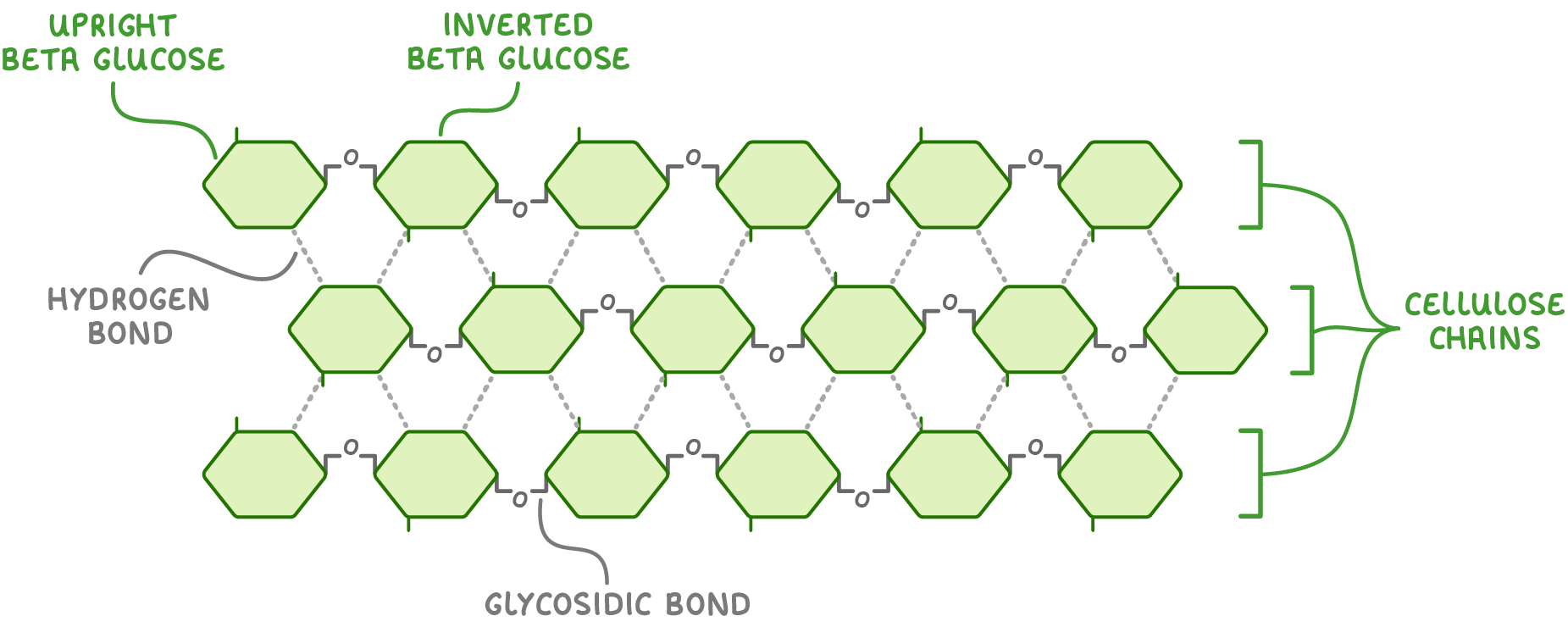 |
Cellulose chains, microfibrils, and macrofibrils Multiple cellulose chains become tightly cross linked via hydrogen bonds to form bundles called microfibrils. These microfibrils join together to make macrofibrils which combine to make strong cellulose fibres in the plant cell wall. 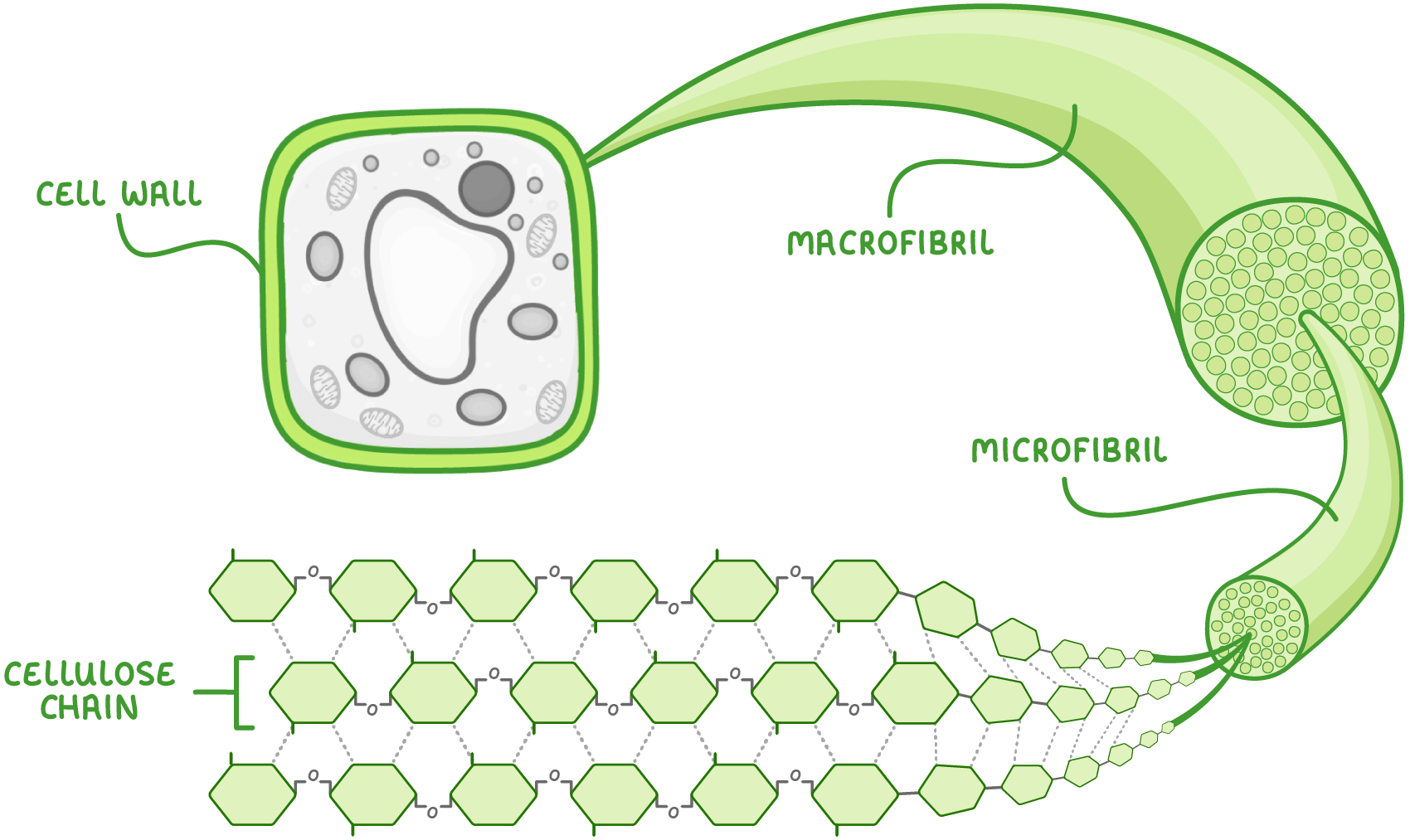 |
Adaptations of cellulose for its role The structure of cellulose is well adapted to its role:
|
Comparing starch, glycogen, and cellulose 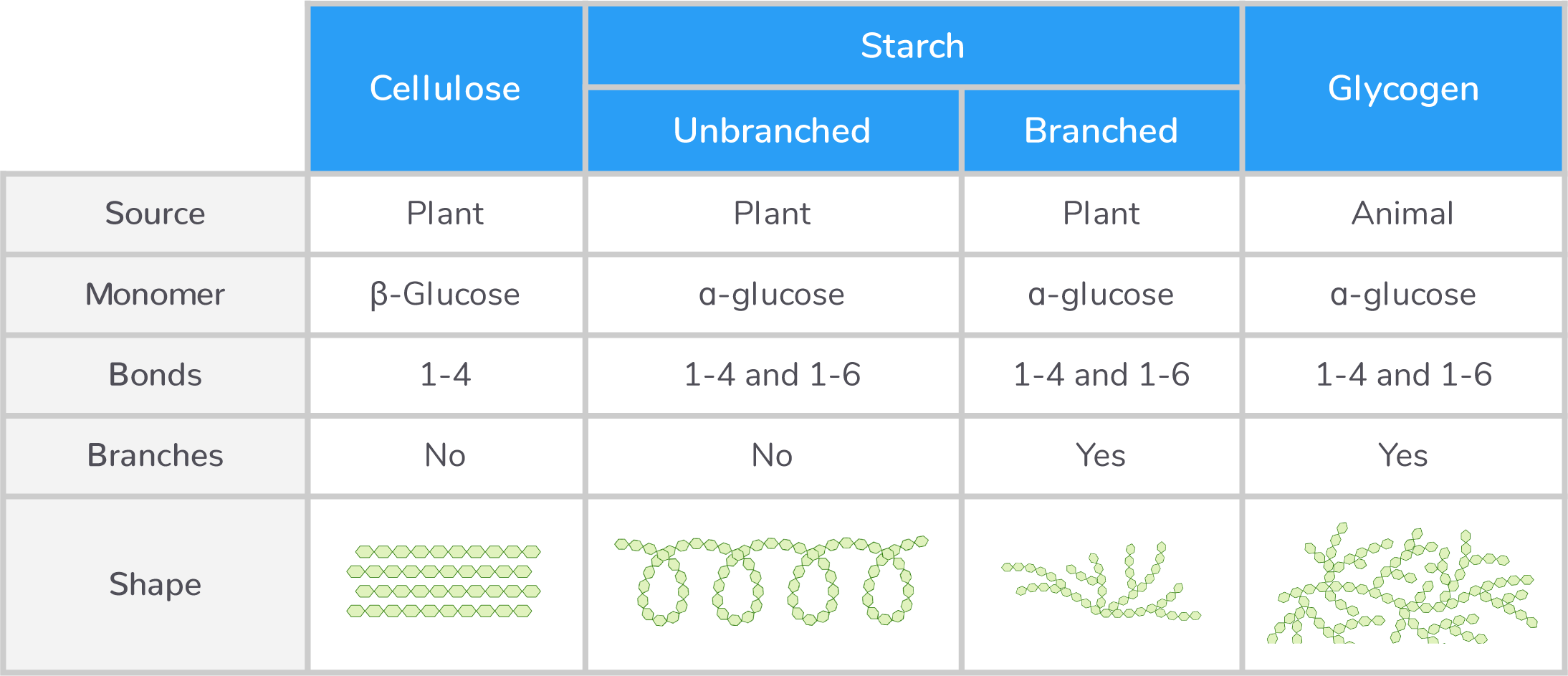 |