Nitrogen Cycle
This lesson covers:
- The different processes involved in the nitrogen cycle
- The different chemical forms nitrogen takes throughout the nitrogen cycle
- The names and roles of the different microorganisms involved in the nitrogen cycle
Nitrogen cycle - overview |
The nitrogen describes how nitrogen is recycled within an ecosystem. It covers the movement of nitrogen between the air, the soil, and the organisms in an environment. The nitrogen cycle also looks at how nitrogen is converted between different chemical forms by different processes, as it moves throughout the cycle. |
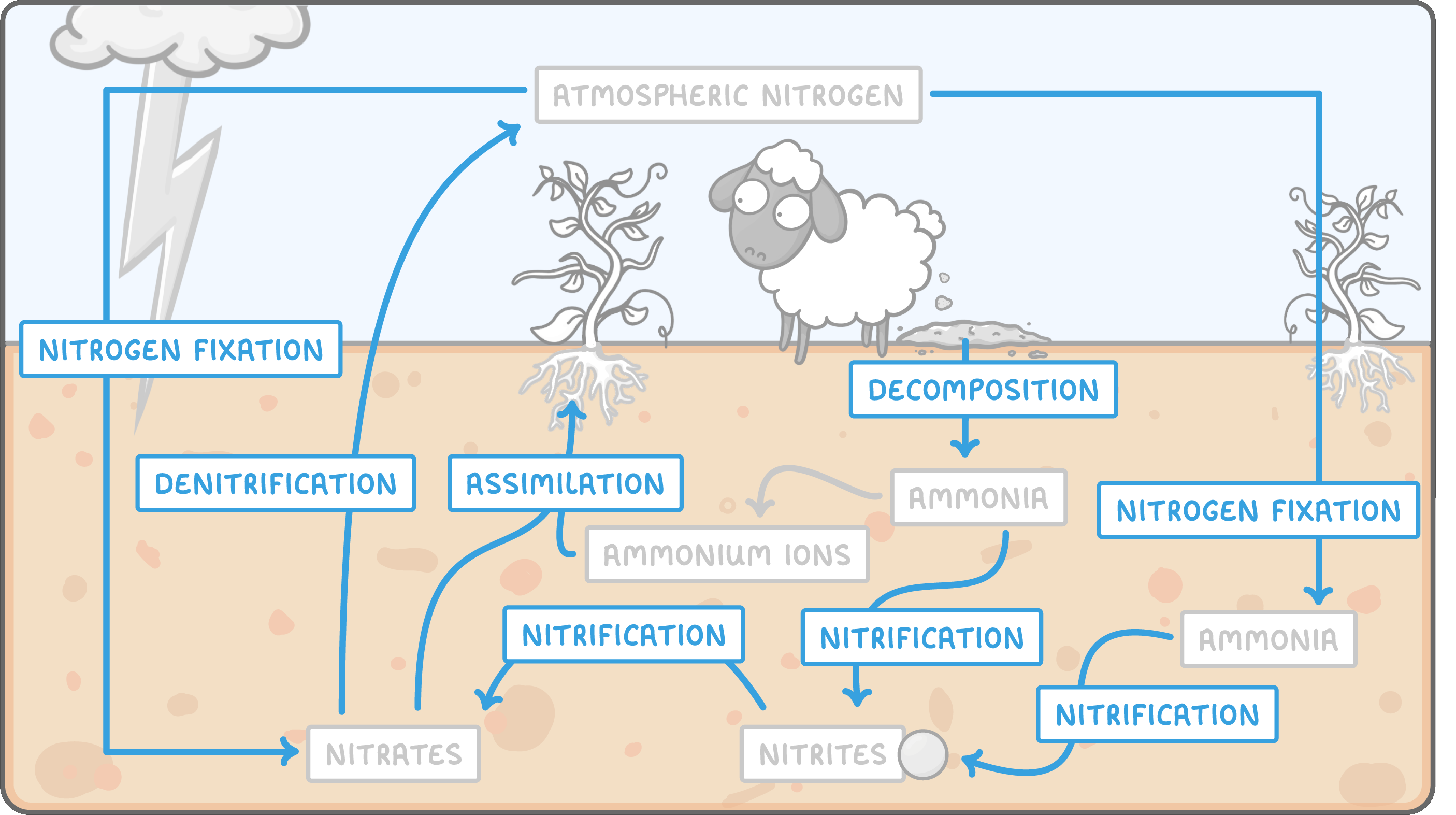 The nitrogen cycle is quite complex. It can be easier to learn if you think about it as 5 different processes: nitrogen-fixation, nitrification, decomposition, assimilation, and denitrification.
|
Nitrogen fixation 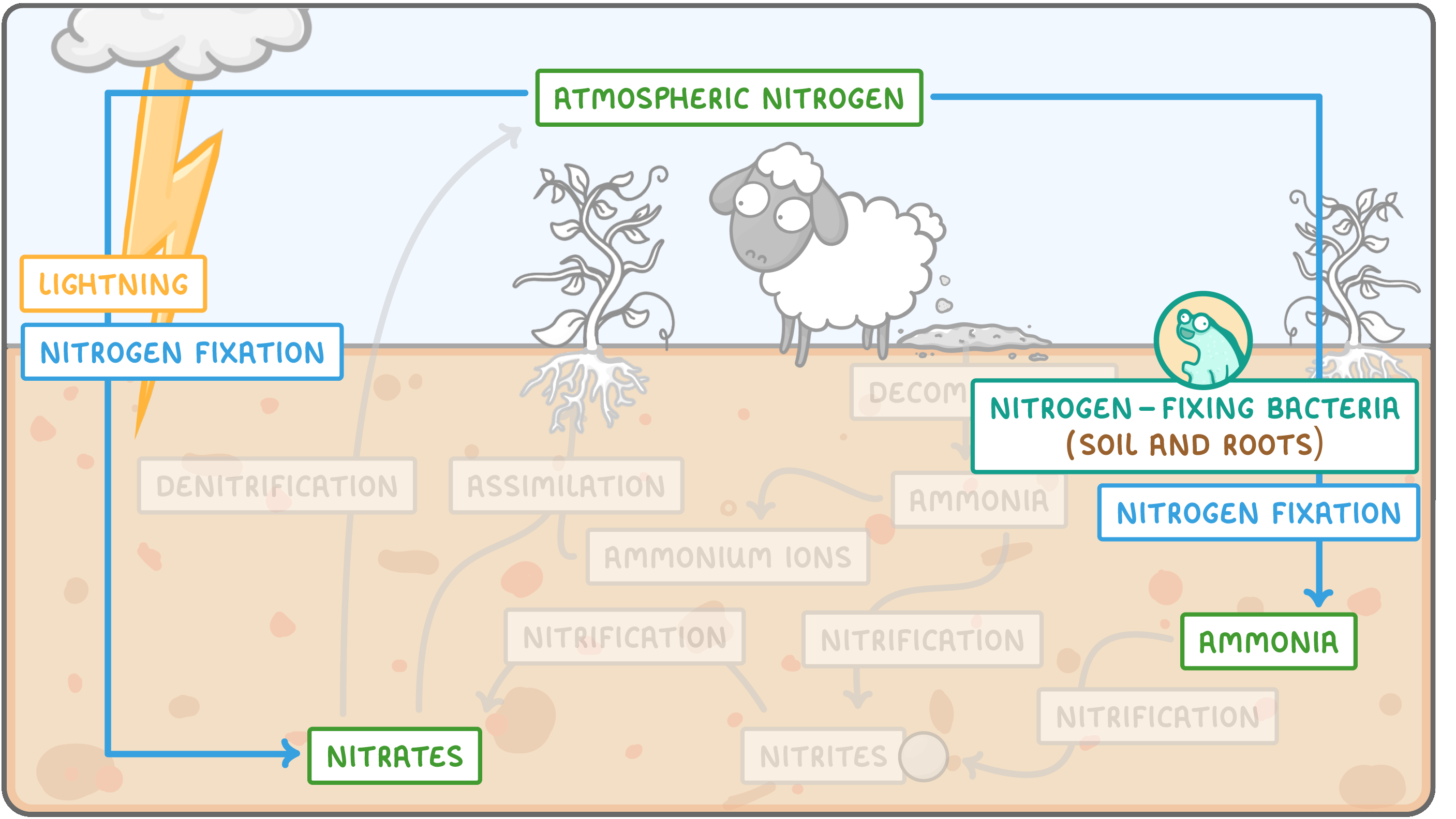 Nitrogen fixation is the process by which atmospheric nitrogen in the air is converted to nitrates and ammonia in the soil. Nitrogen fixation happens in two ways:
|
Nitrification 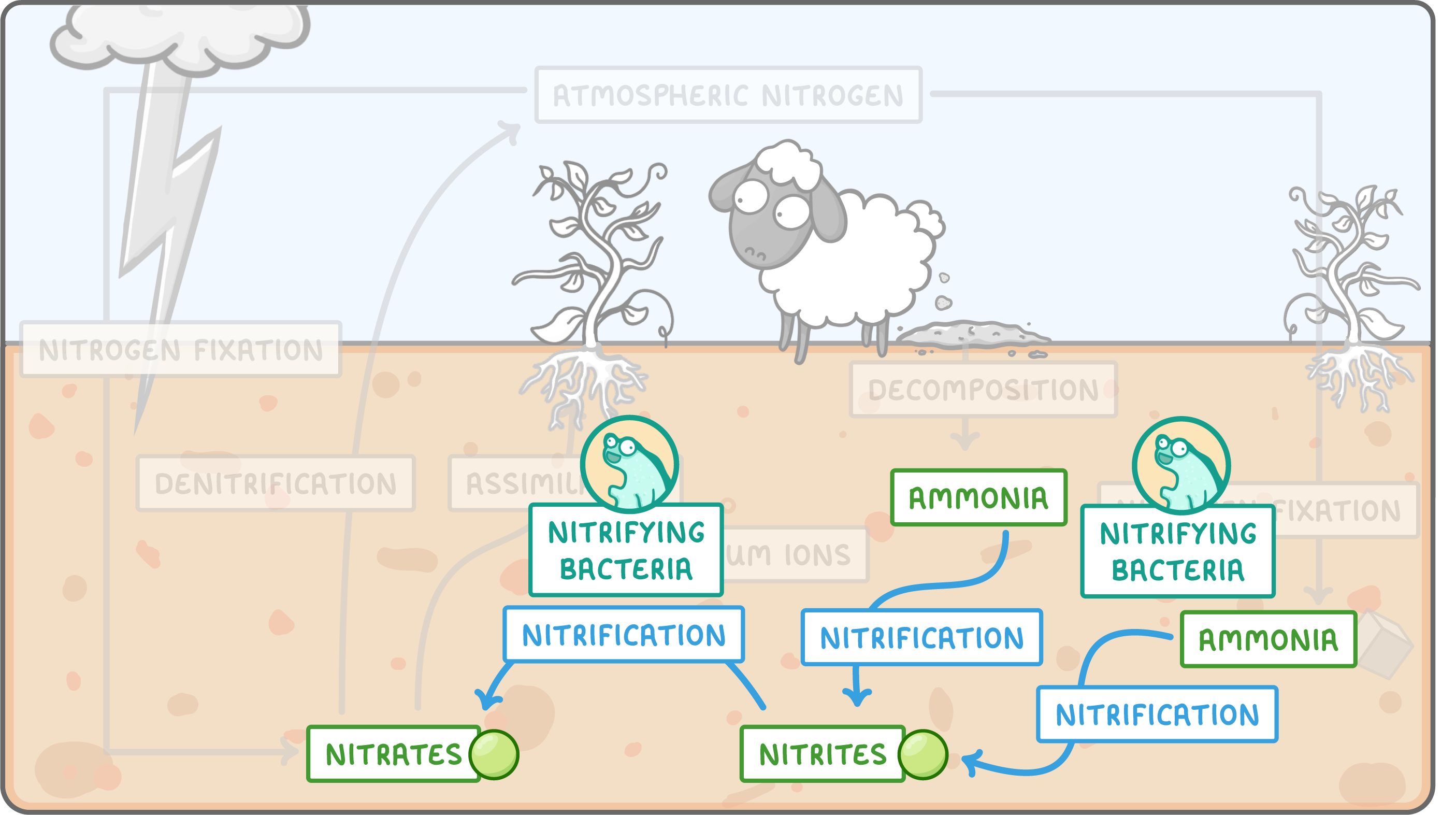 Nitrification is carried out by nitrifying bacteria in the soil, and consists of two steps:
|
Assimilation 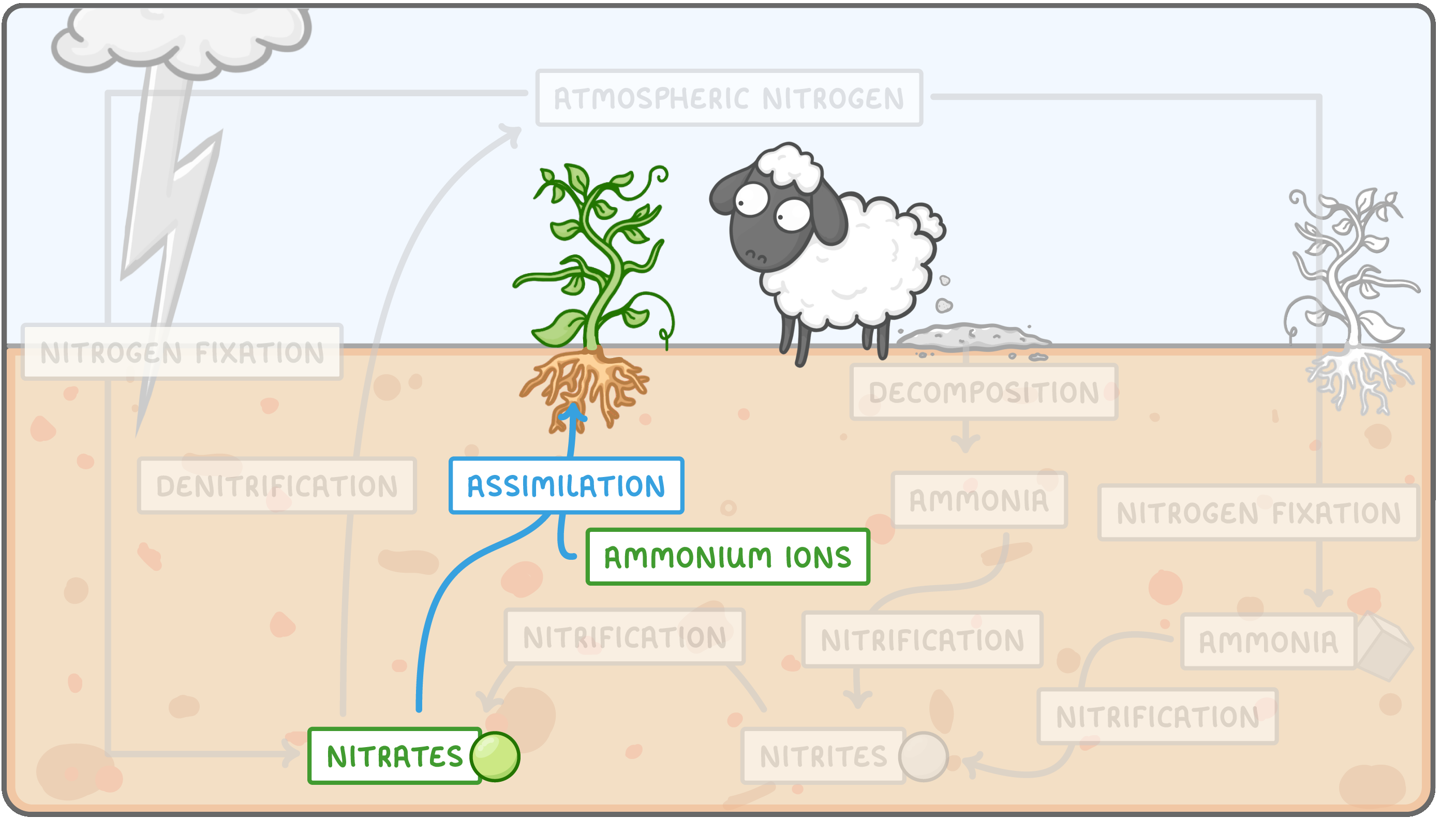 Assimilation is the process by which plants absorb nitrates and ammonium ions, and use them to create biological molecules such as proteins. These biological molecules form part of the plant's tissues. This is how nitrogen enters the food chain, and when animals eat plants they assimilate these nitrogen containing compounds (e.g. proteins) into their own bodies. |
Decomposition 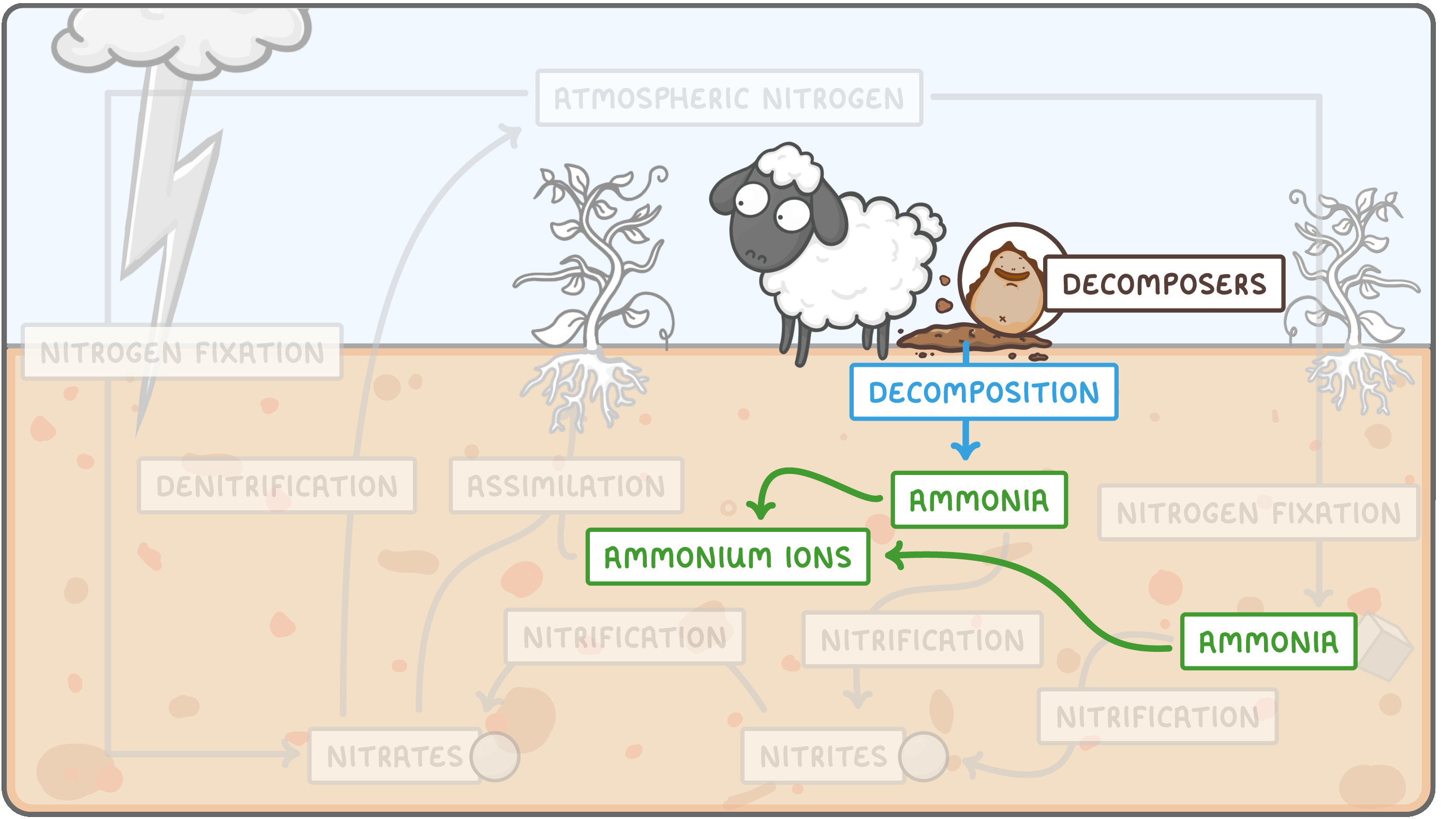 Decomposition occurs when decomposers feed on the waste excretions of animals, and the rotting corpses of dead plants and animals. During this process they convert urea and proteins into ammonia. This is important because the ammonia in the soil forms ammonium ions, which is one of few the nitrogen-containing compounds plants can absorb and assimilate. Note that the some of the ammonia produced by nitrogen fixation is also converted to ammonium ions. (In exams, mark schemes often allow both 'ammonia' and 'ammonium ions' to be used interchangeably, so don't worry if you find it tricky to remember the difference) |
Denitrification  In denitrification, denitrifying bacteria in the soil convert nitrates back into atmospheric nitrogen. Denitrification is bad for plants because it means there are fewer nitrates in the soil, reducing the amount available for the plants to absorb and assimilate. |
Nitrogen cycle - summary & recap |
Don't worry if you find the nitrogen cycle confusing at this point. Read the following summary of the key learning points, and then try reading through the lesson again. Nitrogen cycle summary
|
Which two of the following biological molecules contain nitrogen?
Proteins
Starch
DNA
Fatty acids
|
True or false? Nitrogen-fixing bacteria are found in lightning.
True
False
|
What is the name of the process that converts ammonia to nitrite, and then to nitrates?
Denitrification
Assimilation
Decomposition
Nitrification
|
What is the name of the process that converts nitrates to atmospheric nitrogen?
Assimilation
Nitrification
Nitrogen fixation
Denitrification
|
Which process describes how plants use nitrogen to build biological molecules, that the plants then incorporate into their bodies?
Nitrification
Assimilation
Nitrogen fixation
Denitrification
|
In which two places are nitrogen-fixing bacteria found?
Soil
Ammonium solution
Pieces of shell
Roots of some plants
|
True or false? Plants can absorb atmospheric nitrogen.
True
False
|
The microorganisms responsible for converting atmospheric nitrogen to ammonia/ammonium ions, are called bacteria.
|