Welcome to the Quiz!
This quiz contains 6 questions from a mix of 1 subtopics.
When Democritus first conceived of atomic theory, around 500 BC, how did he describe atoms?
(Select all that apply)
The smallest possible unit of matter
Small spheres
Separated from each by empty space
Tiny living creatures
|
rocks / cubes / spheres / atoms / elements
In the 1800's John Dalton described atoms as solid , and suggested that different types of spheres make up the different .
|
In 1897 J J Thomson theorised that an atom consists of a ball of positive charge, with negative electrons mixed throughout it. What do we call this model?
Quantum Model
Nuclear model
Atomic model
Plum pudding model
|
How Rutherford developed the nuclear model
beta / deflected / positive / alpha / negative
In Rutherford's experiments, particles were fired at a thin sheet of gold foil.
Most particles passed through, but some were off course.
This caused him to hypothesise that there was a dense region of charge at the centre of the atom that repelled the alpha particles.
As a result he developed the nuclear model of the atom, in which there was a central positive nucleus, surround by electrons.
|
One issue with Rutherford's nuclear model was that the atom should collapse as the negative electrons would be attracted to the positive nucleus, causing them to rush inwards.
In response to this, in 1913 Bohr suggested that electrons ___________________, which kept the atom from collapsing.
are actually positively charged
have magnetic repulsion
orbit the nucleus in shells
|
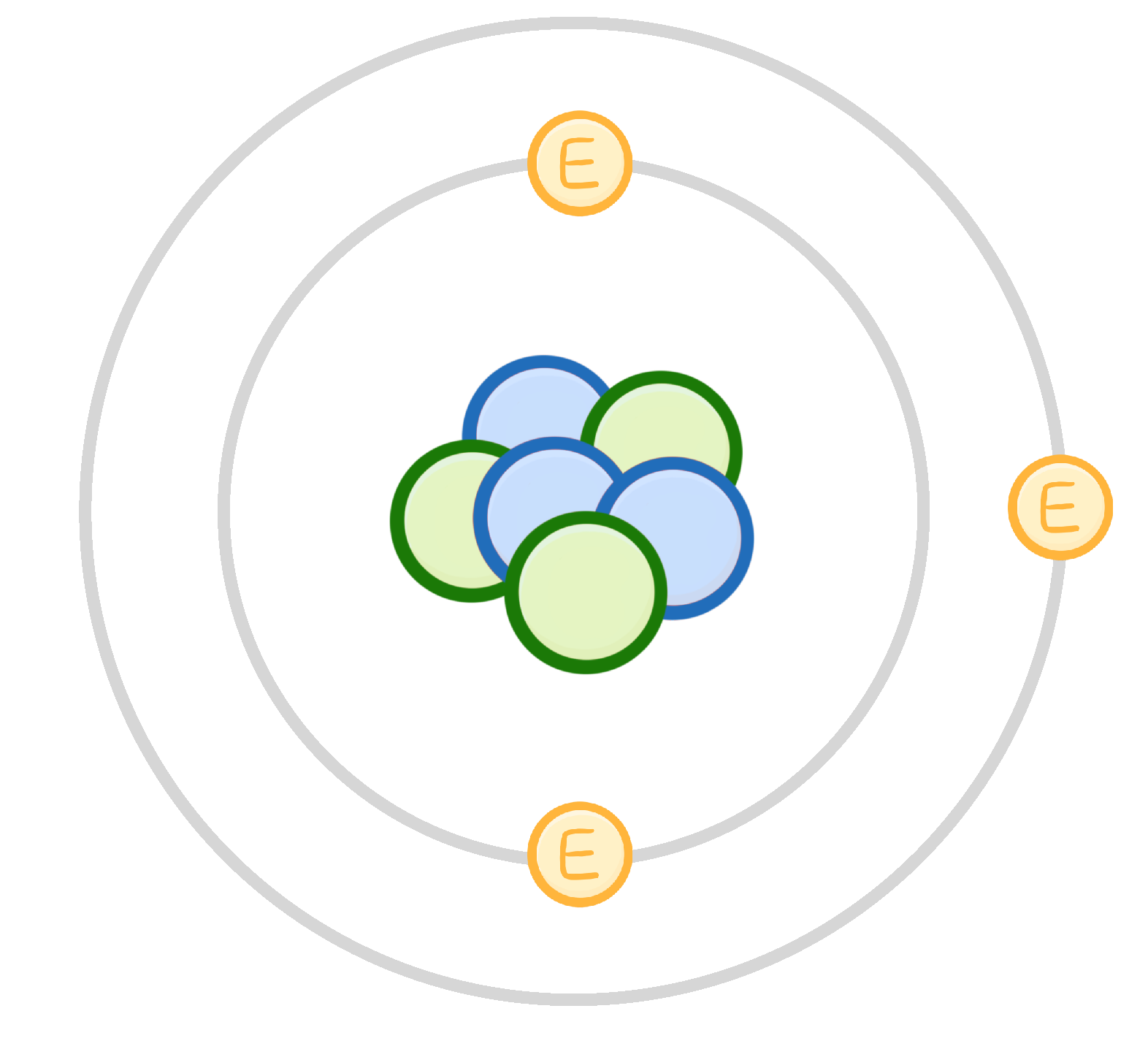
In the 20th century, Chadwick discovered neutral particles in the atomic nucleus. What are these particles called?
Electrons
Protons
Neutrons
|