Welcome to the Quiz!
This quiz contains 7 questions from a mix of 1 subtopics.
Which two the following represent an alpha particle?
|

The above nuclear equation shows thorium (Th) undergoing alpha decay.
Fill in the missing atomic mass (A)and atomic number (B):
A:
B:
|
Which of the following represent a beta particle?
|
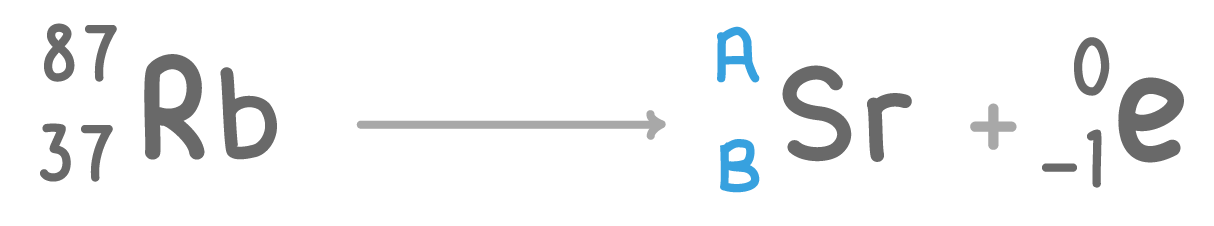
The above nuclear equation shows rubidium (Rb) undergoing beta decay.
Fill in the missing atomic mass A and atomic number B:
A:
B:
|
Which of the following represents gamma radiation?
|
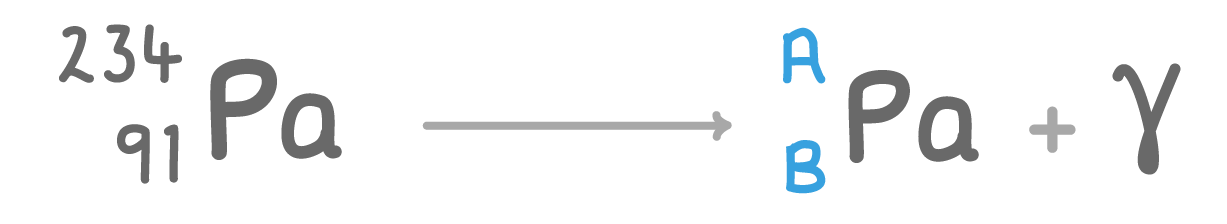
The above nuclear equation shows protactinium (Pa) undergoing gamma ray decay.
Fill in the missing atomic mass A and atomic number B:
A:
B:
|
element / molecule / protons / neutrons
The nucleus of any can be represented in the following form:
This is the nucleus of the element carbon.
- The C is the elemental (or chemical) symbol
- The 12 is the 'mass number', which is the number of protons and neutrons combined
- The 6 is the 'atomic number', which is the number of . This can also be thought of as the particle's charge.
|