Welcome to the Quiz!
This quiz contains 10 questions from a mix of 1 subtopics.
Which subatomic particles make up the nucleus of an atom?
(Select all that apply)
Electrons
Neutrons
Protons
|
Which of the following is true?
Electrons have positive charge and neutrons have negative charge
Electrons have no charge and protons have positive charge
Neutrons have no charge and protons have positive charge
Protons have positive charge and neutrons have negative charge
|
What are the charges of the subatomic particles?
Electron:
Proton:
Neutron:
|
What are the relative masses of the following subatomic particles?
Proton:
Neutron:
|
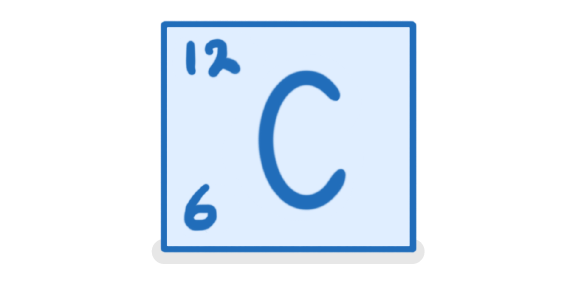
The above image shows an element in the periodic table. State the:
Name of the element:
The mass number:
The atomic number:
|
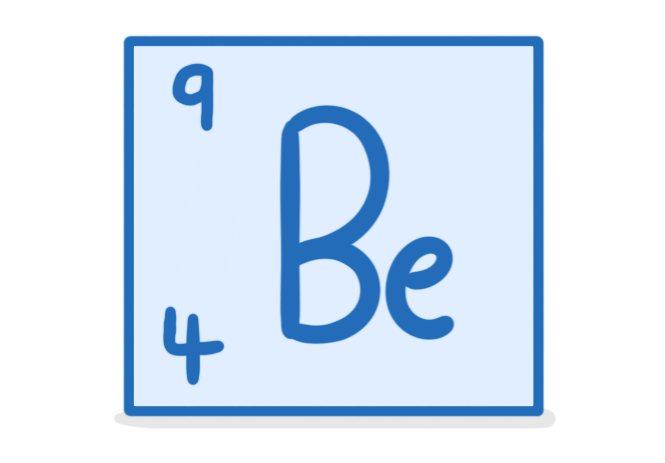
The above image shows the element 'beryllium'. For an atom of beryllium, state the:
Number of protons:
Number of electrons:
Number of neutrons:
|
electrons / protons / neutrons
Isotopes are atoms with the same number of but different number of .
|
Which of the following are plausible isotopes of carbon?
|
stable / unstable / gamma / theta
Some isotopes are and can decay into other elements. Decay involves emitting radiation such as alpha, beta, and radiation.
|
Electrons, shells, and ionisation
absorb / emit / shells / ionisation / radiation / nearer / further
- Electrons orbit the nucleus at specific energy levels which are also called .
- The shells get progressively from the nucleus and increase in energy level.
- Electrons can electromagnetic radiation and jump to a higher energy level / shell. In these cases we say that the electron has become 'excited'.
- Excited electrons can later fall back to a lower energy level, and electromagnetic radiation in the process.
- Electrons can also absorb enough energy to leave the atom altogether, this is known as .
|