Welcome to the Quiz!
This quiz contains 7 questions from a mix of 1 subtopics.
When kept at a constant temperature, the pressure and volume of a gas are proportional.
|
For a gas at a constant temperature, which statements are true?
(Select all that apply)
When volume decreases, pressure increases
When volume increases, pressure increases
When volume increases, pressure decreases
When volume decreases, pressure decreases
|
Which is the correct formula for the relationship between pressure and volume, at constant temperature?
|
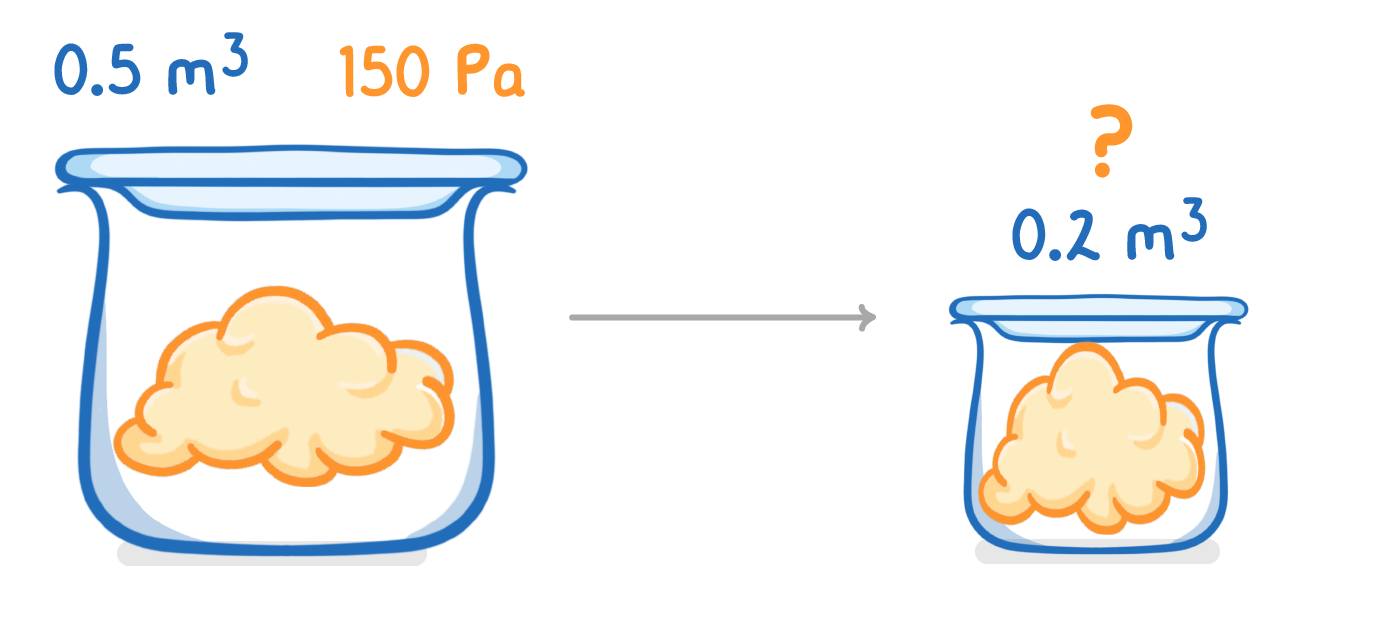
A gas occupies a volume of 0.5 m3 at a pressure of 150 Pa.
Calculate the pressure exerted by the gas if the volume is compressed to 0.2 m3?
(Assume constant temperature)
Pa
|
A gas occupies a volume of 0.3 m3 at a pressure of 50 Pa.
The pressure increased to 150 Pa, what is the new volume?
(Assume constant temperature)
m3
|
A gas initially occupied a volume of 10 L at an unknown pressure.
The gas was then compressed to 2 L, and measured as having a pressure of 20 kPa.
Calculate the original pressure before the gas was compressed.
(Assume constant temperature)
kPa
|
A solid container is filled with a gas at a pressure of 100 kPa and a temperature of 150 K. The container (and gas inside it) is then heated to a temperature of 240 K.
Assuming a constant volume, calculate the new pressure after the gas has been heated.
kPa
|