Welcome to the Quiz!
This quiz contains 17 questions from a mix of 1 subtopics.
Atoms consist of a central containing protons and . The nucleus is surrounded by arranged in energy levels (also called shells). Atoms have no electric charge because they contain the same number of and electrons.
|
What is the relative mass of a neutron?
0
0.0005
1
2
|
What is the charge of a proton?
-1
0
+1
|
are neutral, so have a charge of 0.
|
What is the charge of an electron?
-1
0
+1
|
What do we call the number labelled above?
number
|
Atoms and ions of the same element always have the same number of .
|
The mass number tells you the number of and neutrons in the of the atom.
|
number = number of protons
|
Using the nuclear symbol given above, fill in the following missing values:
Atomic number:
Number of protons:
|
Using the nuclear symbol given above, work out the number of electrons.
|
Using the nuclear symbol given above, fill in the following missing values:
Mass number:
Number of neutrons:
|

The above nuclear symbol represents uranium-235, fill in the missing values:
Atomic number:
Mass number:
Number of protons:
Number of neutrons:
Number of electrons:
|

The above nuclear symbol represents uranium-238, fill in the missing values:
Mass number:
Number of neutrons:
|
Using the data in the table above, work out the missing values.
Atom or ion:
Number of electrons:
|
Using the data in the table above, work out the missing values.
Atom or ion:
Number of electrons:
|
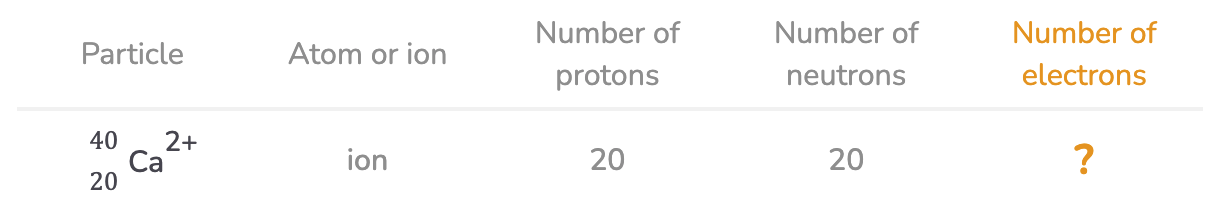
Using the data in the table above, work out the missing values.
Number of electrons:
|