Welcome to the Quiz!
This quiz contains 27 questions from a mix of 1 subtopics.
Atoms consist of a central containing protons and . The nucleus is surrounded by arranged in energy levels (also called shells). Atoms have no electric charge because they contain the same number of and electrons.
|
What is the relative mass of a neutron?
0
0.0005
1
2
|
What is the charge of a proton?
-1
0
+1
|
are neutral, so have a charge of 0.
|
What is the charge of an electron?
-1
0
+1
|
What do we call the number labelled above?
number
|
Atoms and ions of the same element always have the same number of .
|
The mass number tells you the number of and neutrons in the of the atom.
|
number = number of protons
|
Using the nuclear symbol given above, fill in the following missing values:
Atomic number:
Number of protons:
|
Using the nuclear symbol given above, work out the number of electrons.
|
Using the nuclear symbol given above, fill in the following missing values:
Mass number:
Number of neutrons:
|

The above nuclear symbol represents uranium-235, fill in the missing values:
Atomic number:
Mass number:
Number of protons:
Number of neutrons:
Number of electrons:
|

The above nuclear symbol represents uranium-238, fill in the missing values:
Mass number:
Number of neutrons:
|
Using the data in the table above, work out the missing values.
Atom or ion:
Number of electrons:
|
Using the data in the table above, work out the missing values.
Atom or ion:
Number of electrons:
|
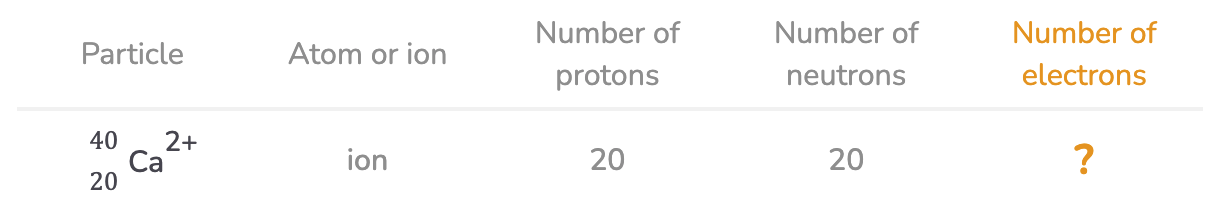
Using the data in the table above, work out the missing values.
Number of electrons:
|
Match the name of the atomic model to the scientist that proposed it.
|
Name the sub-atomic particle discovered by James Chadwick.
|
Select ALL of the contributions made by Niels Bohr to the development of the atomic model.
discovered the nucleus
discovered the proton
explained why atoms didn't collapse in on themselves
discovered the neutron
proposed that electrons orbit the nucleus in fixed shells
|
Select ALL the contributions made by Ernest Rutherford to the development of the atomic model.
proposed that electrons orbit the nucleus in fixed shells
discovered the nucleus
discovered the proton
explained why atoms didn't collapse in on themselves
discovered the neutron
|
Select the best conclusion that can be made from Rutherford's observation that some of the alpha particles were deflected at large angles,
most of the atom is empty space
most of the mass is concentrated in a central nucleus
all of the positive charge is concentrated in a central nucleus
|
Select the best conclusion that can be made from Rutherford's observation that most of the alpha particles passed straight through the gold foil undeflected.
most of the mass is concentrated in a central nucleus
all of the positive charge is concentrated in a central nucleus
most of the atom is empty space
|
What type of particle did Rutherford fire at the thin sheet of gold?
protons
neutrons
alpha particles
beta particles
|
Which sub-atomic particle was discovered by JJ Thomson?
neutron
electron
proton
|
Dalton believed atoms were spheres.
|
Did Dalton suggest that the atoms of a given element were identical or different to each other?
identical to each other
different to each other
|