Welcome to the Quiz!
This quiz contains 13 questions from a mix of 1 subtopics.
An electrolysis cell has two electrodes. What is the name of the positive electrode?
Cathode
Anode
|
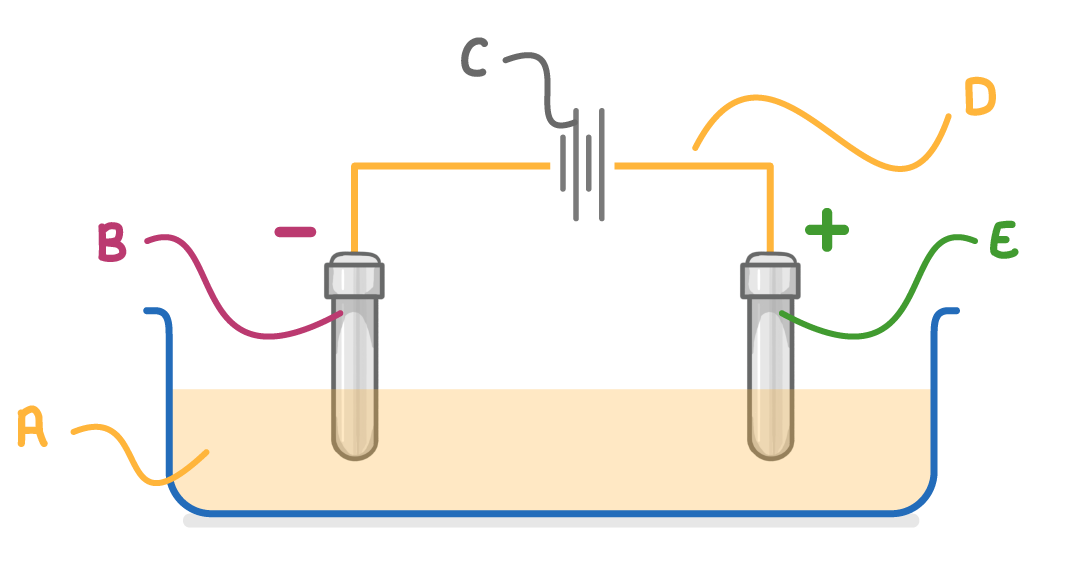
What is the name of the liquid indicated by letter A on the diagram above?
|

In electrolysis, which direction do the electrons travel?
Cathode ➔ Anode
Anode ➔ Cathode
|
Which ions are attracted to the negative electrode (cathode) during electrolysis?
Negative ion
Positive ions
|
The electrodes in an electrolysis cell are normally made of inert carbon. What does the term 'inert' mean?
It cannot conduct electricity
It is chemically reactive and involved in the reaction
It is unreactive, so will not take place in the reaction.
|
In electrolysis, is the ion that gains electrons oxidised or reduced?
Oxidised
Reduced
|
In the electrolysis of molten lead bromide, what is the product at the anode?
Bromine
Lead
Oxygen
|
In terms of electrons, what does oxidation mean?
Loss of electrons
Gain of electrons
|
In electrolysis, why does the compound you're trying to separate need to be molten or dissolved?
|
In electrolysis, which electrode would chloride ions (Cl-) be attracted to?
Cathode
Anode
|
On the diagram above, which letter indicates the cathode?
A
B
C
D
E
|
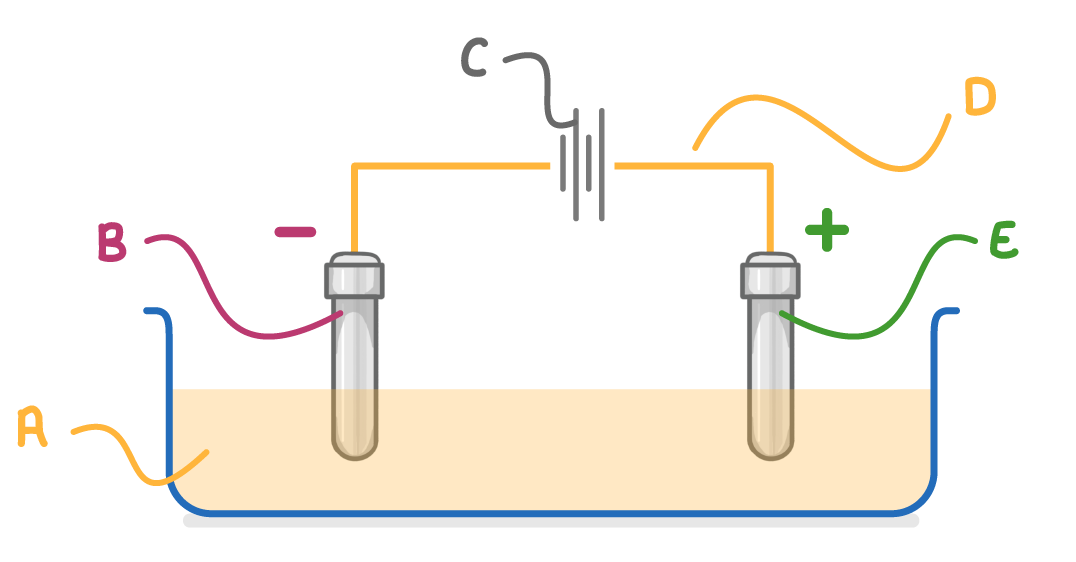
On the diagram above, which letter indicates the anode?
A
B
C
D
E
|
On the diagram above, which letter indicates the electrolyte?
A
B
C
D
E
|