Welcome to the Quiz!
This quiz contains 6 questions from a mix of 1 subtopics.
When metal ions are heated they start to ________ light.
reflect
absorb
emit
|
How flame emission spectroscopy works
light / wavelengths / ions / identify / spectroscope / flame / unique
- First, metal are heated until they emit light.
- The light is detected by a which can distinguish between the individual of light emitted.
- As each metal ion emits a unique combination of wavelengths it will produce a line spectrum.
- This allows us to an unknown metal cation by comparing its line spectrum to those of known metal cations in a data bank.
|
What does the intensity of a line spectrum indicate about a cation?
Its reactivity
Its concentration
Its group on the periodic table
|
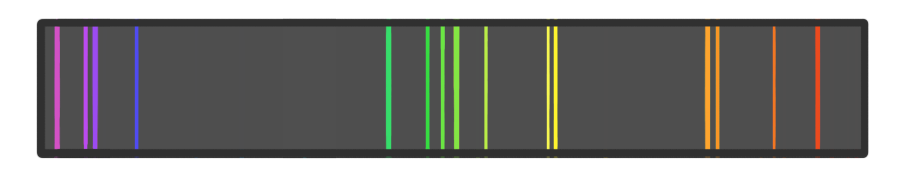
An unknown substance was analysed using flame emission spectroscopy, and the above line spectrum was detected.
Which cation is present in the substance?
(Note: these are not accurate representations of the emission spectrums for the elements labelled)
|
spectrum / anions / cations
If a substance contains multiple , all of their line spectrums will be combined into a single line .
|
What are the three main benefits of instrumental methods of chemical analysis over manual methods?
Higher sensitivity
Higher cost
Higher accuracy
Faster test
|