Welcome to the Quiz!
This quiz contains 9 questions from a mix of 1 subtopics.
positive / negative / electrostatic / ionic / covalent
Metals normally form ions which have a charge, while non-metals form ions with a charge.
Ions are attracted to other ions with the opposite charge, due to forces.
|
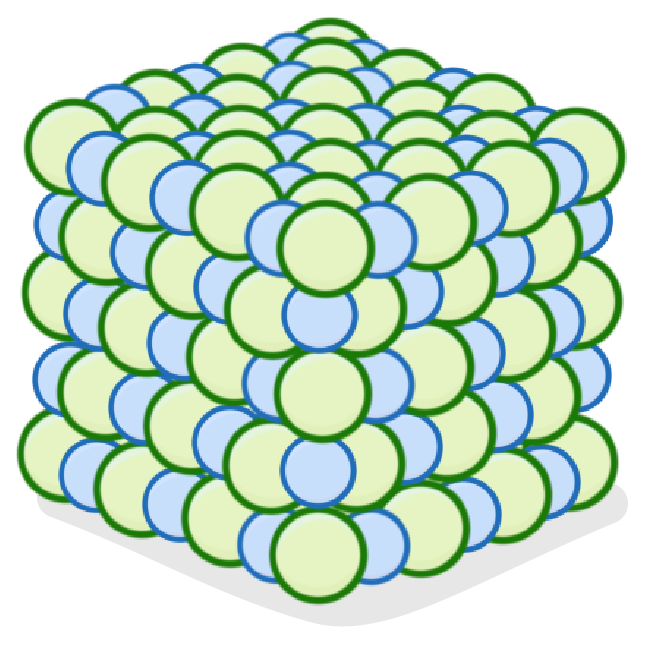
Ionic structures involve many ions bonded together via bonds.
The solid arranges itself into a regular 3D structure known as a la.
|
Select three properties that ionic compounds have:
Weak strength bonds
Low melting and boiling points
Conduct electricity when liquid
High melting and boiling points
Conduct electricity when solid
High strength bonds
|
Why can ionic compounds only conduct electricity when in liquid/molten, or aqueous form?
Solid ionic compounds do not contain charged particles
Water is required to conduct electricity
The ions are free to move and carry charge
The negative ions can approach other negative ions
|
strong / weak / high / low / energy / water
Ionic compounds have relatively melting and boiling points.
This is because ionic bonds are very , and a high amount of is required to break the bonds.
|
Select the correct formula for the ionic compound potassium chloride:
K2Cl
KCl
NaCl
KCl2
|
Select the correct formula for a nitrate ion:
NO3
NO3+
NO3-
N3-
|
Potassium ions and carbonate ions can form an ionic compound.
Potassium ions have a + charge, while carbonate ions have a - charge.
This means every carbonate ion will ionically bond with potassium ions.
|
What is the chemical formula for the compound calcium sulfate?
CaSO4
Ca(SO4)2
CaSO42+
Ca2SO4
|