Welcome to the Quiz!
This quiz contains 9 questions from a mix of 1 subtopics.
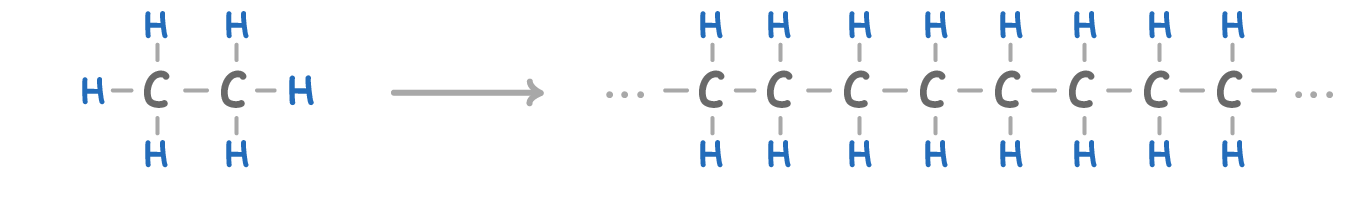 |
As the chain length (number of carbon atoms) of alkanes increases, they become: More viscous Less viscous
|
More volatile Less volatile
|
More flammable Less flammable
|
|
Increasing the chain length (number of carbons) of alkanes leads to:
(Select all that apply)
Higher boiling point
Lower boiling point
Lower melting point
Higher melting point
|
ethanol / oxygen / water / nitrogen
What is the word equation for the complete combustion of a hydrocarbon?
hydrocarbon + ➔ carbon dioxide +
|
C7H16 + ?O2 ➔ ?CO2 + H2O The above combustion reaction needs to be balanced. Start by balancing the number of H2O molecules so that the number of hydrogen atoms is equal on both sides of the equation.
|
C7H16 + ?O2 ➔ CO2 + 8H2O Next, balance the number of CO2 molecules by making sure there are an equal number of carbons on both sides of the equation.
|
C7H16 + O2 ➔ 7CO2 + 8H2O Finish by balancing the number of O2 molecules, so that there are an equal number of oxygen atoms on both sides of the equation.
|
|

Is combustion an exothermic or endothermic reaction?
Exothermic
Endothermic
|
Please balance the following combustion equation:
C3H8 + O2 ➔ CO2 + H2O
|
During a combustion reaction, are carbon and hydrogen oxidised or reduced?
Oxidised
Reduced
|
Balance the following combustion equation:
C2H6 + O2 ➔ CO2 + H2O
|
For alkanes, increasing chain length (increasing the number of carbons) leads to molecules that are:
(Select all that apply)
More flammable
More volatile
Less flammable
Less volatile
|