Welcome to the Quiz!
This quiz contains 15 questions from a mix of 1 subtopics.
Organic chemistry is about molecules that contain which element?
Carbon
Iron
Magnesium
Argon
|
Hydrocarbons only contain which two elements?
Hydrogen
Carbon
Oxygen
Helium
|
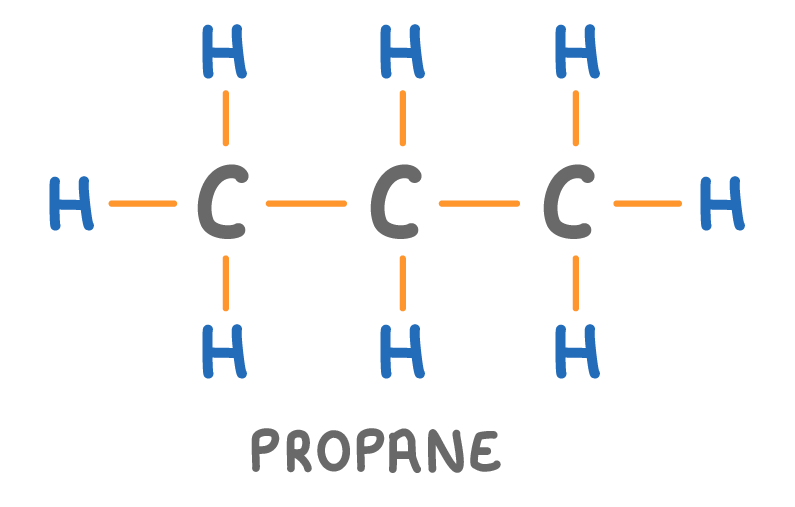
Is the molecule above a hydrocarbon?
Yes
No
|
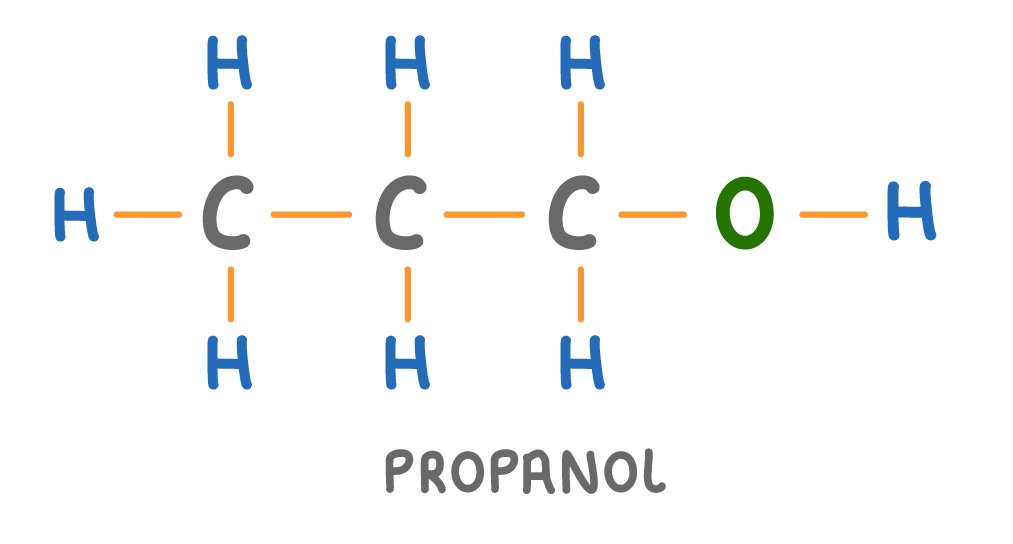
Is the molecule above a hydrocarbon?
Yes
No
|
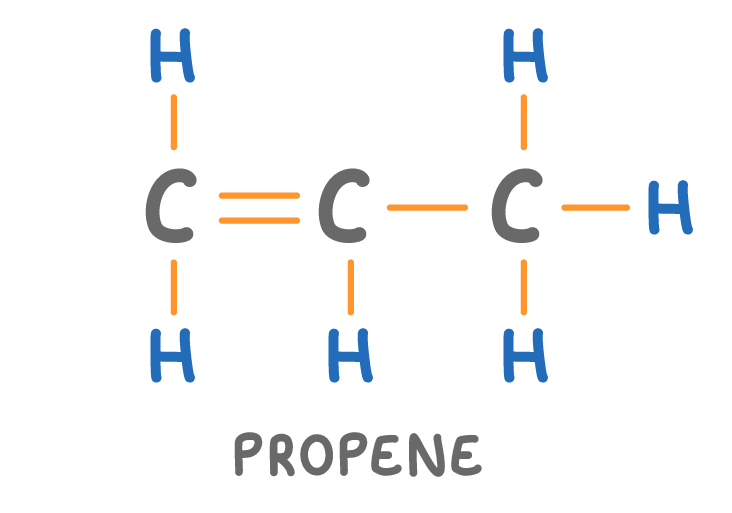
Is the molecule above a hydrocarbon?
Yes
No
|
What is the definition of a homologous series?
|
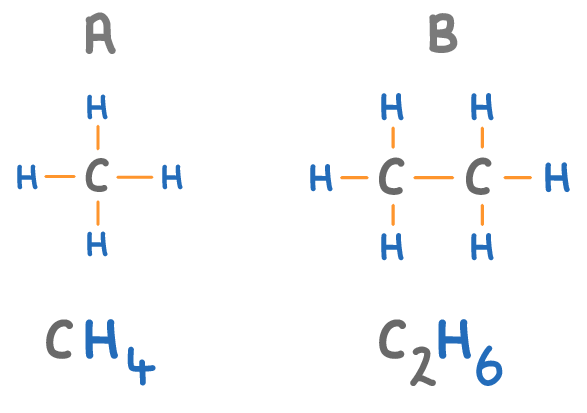
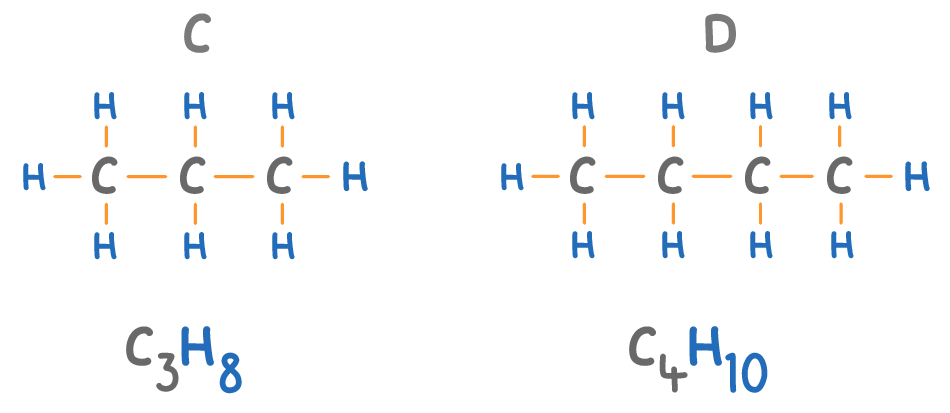
The names of alkanes always end in 'ane'.
Match the molecules A to D on the diagram above with their names:
Methane:
Butane:
Ethane:
Propane:
|
Which alkane is shown above?
Propane
Butane
Ethane
Methane
|
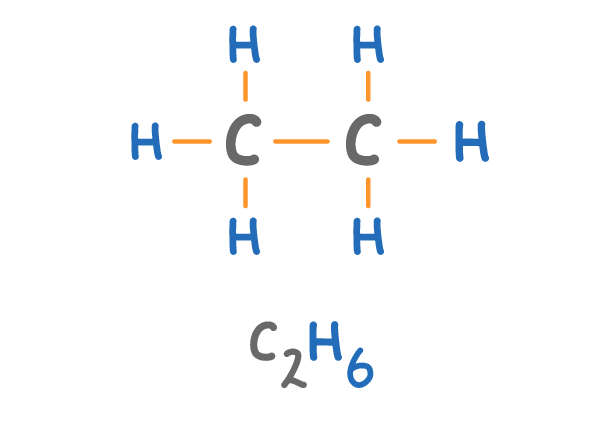
Which alkane is shown above?
Butane
Ethane
Methane
Propane
|
Which alkane is shown above?
Propane
Methane
Butane
Ethane
|
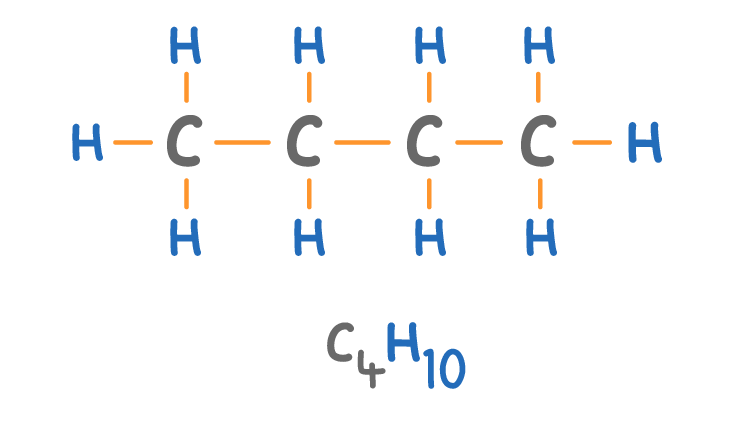
Which alkane is shown above?
Propane
Ethane
Methane
Butane
|
Each homologous series has its own general formula, which allows you work out how many atoms of each element a molecule will have.
What is the general formula for alkanes?
(Remember that 'n' represents the number of carbon atoms)
CnH2n
CnH2n+2
CnHn
|
How many hydrogen atoms are there in an alkane with 5 carbon atoms?
hydrogen atoms
|
An alkane only has single covalent bonds. There are no double bonds or triple bonds.
What is another way to describe hydrocarbons with only single bonds?
Saturated
Lone molecules
Unsaturated
|
What is the definition of a hydrocarbon?
|