Welcome to the Quiz!
This quiz contains 12 questions from a mix of 1 subtopics.
Does breaking bonds release energy or require energy?
Require energy
Release energy
|
Is breaking bonds exothermic or endothermic?
Exothermic
Endothermic
|
energy / break / group / mole
The term 'bond energy' refers to the amount of required to one of a particular covalent bond.
|
Bond energy values are always:
Positive
Negative
|
Is forming a bond an exothermic or endothermic process?
Exothermic
Endothermic
|
What type of reaction produces a negative energy change?
For example: -150 kJ/mol, -330 kJ/mol, -855 kJ/mol
An exothermic reaction
An endothermic reaction
|
During a chemical reaction, the bonds of the products must be:
Formed
Broken
|
2H2 + O2 ➔ 2H2O The equation above shows the reaction between hydrogen and oxygen to form water. |
The total energy required to break the bonds of the reactant molecules is 1370 kJ. The total energy released when forming the bonds of the product molecules is 1852 kJ. |
What is the overall energy change of the reaction? kJ/mol
|
Is this an exothermic or endothermic reaction? Exothermic Endothermic
|
|

What is the energy change of the above reaction?
kJ/mol
|
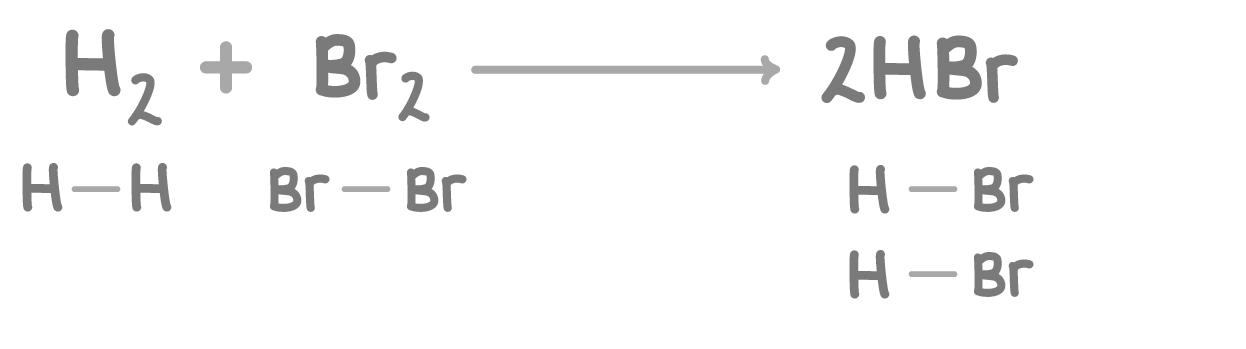
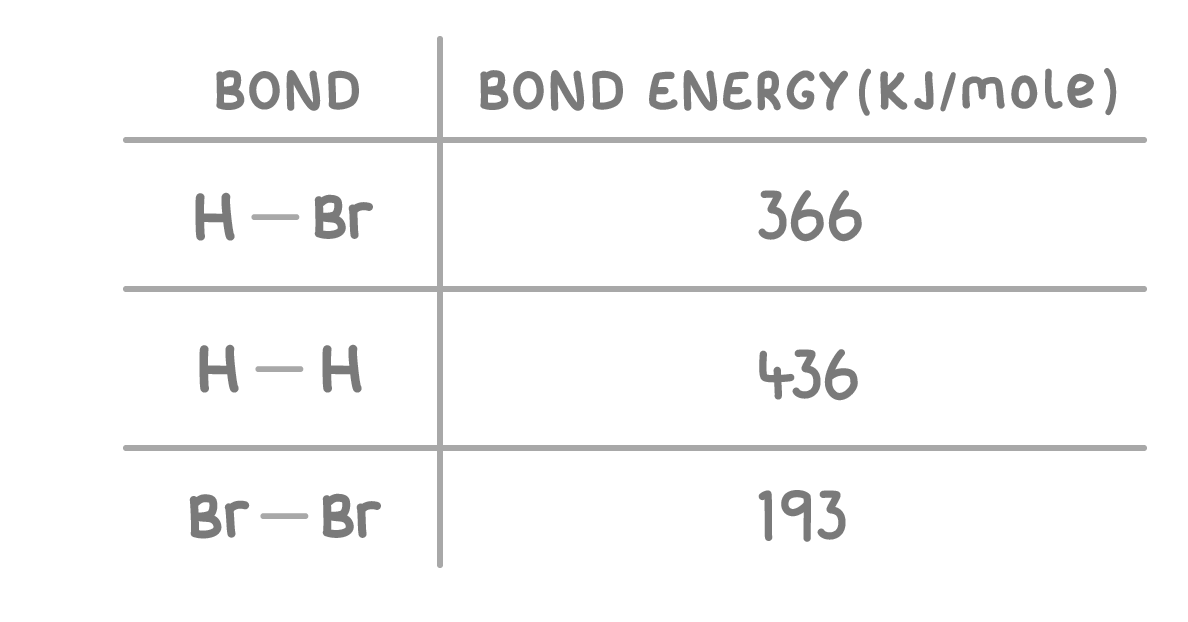
What is the energy change of the above reaction?
kJ/mol
|
What type of reaction produces a positive energy change value?
For example: 20 kJ/mol, 44 kJ/mol, 26 kJ/mol
An endothermic reaction
An exothermic reaction
|
What is the energy change of the above reaction?
kJ/mol
|