Welcome to the Quiz!
This quiz contains 15 questions from a mix of 1 subtopics.
Which theory attempts to explain the three states of matter?
Particle (kinetic) theory
Condensation theory
Nuclear theory
|
The particle model (sometimes also called the kinetic model) has 3 main assumptions. These are that the particles are: |
Large Small
|
Elastic Inelastic
|
Cubes Spheres
|
|
heated / cooled / vibrate / liquid / gas / melts / boils
In solids, strong attractive forces hold the particles in place, so that they can only in position.
As the substance is , the particles gain energy and vibrate faster and faster. Eventually, the particles have so much energy that they can overcome the forces holding them together, and the substance into a .
|
When a solid is heated, energy is transferred to the particles' ________ energy stores, causing them to vibrate faster.
chemical
elastic
kinetic
thermal
|
freeze / boil / melt
As heat is applied to a liquid, the particles gain kinetic energy and move faster. With enough energy they can break the forces of attraction between the molecules. At this point the liquid would into a gas.
|
kinetic / electrostatic / volume / density / pressure
When a gas is heated, the particles gain energy and move faster.
If the gas is trapped within a container that cannot expand, it means that the of the gas is fixed, and so the inside the container increases.
|
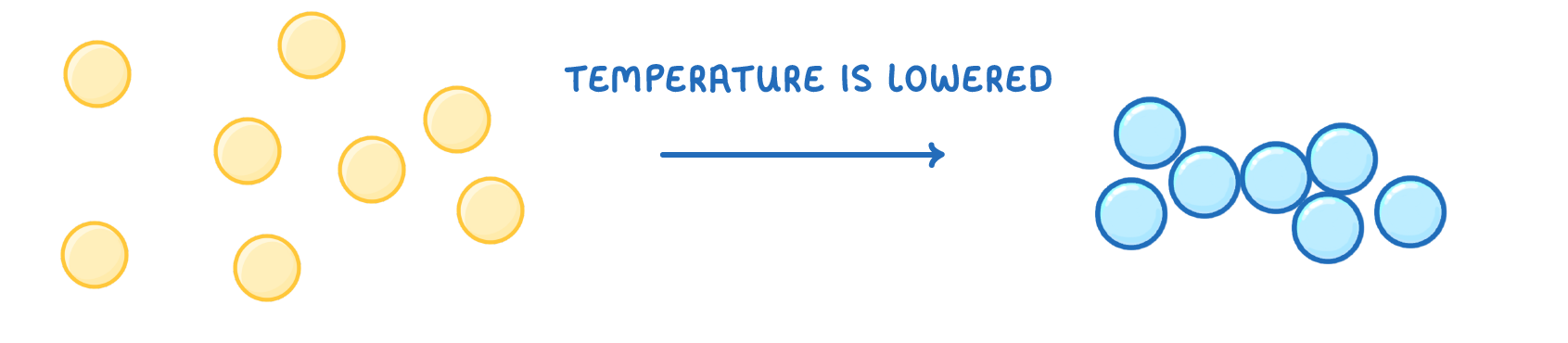
In gases, the particles have enough energy to overcome the attractive forces between them, and so spread out randomly.
If the temperature is lowered, they will no longer be able to overcome these attractive forces, and the particles move closer together, and turn into a liquid.
This process is called ________.
boiling
melting
freezing
condensation
|
The temperature at which a solid converts into a liquid is called the point.
The temperature at which a liquid converts into a gas is called the point.
|

Which state of matter matches each of the descriptions below?
Solid / Liquid / Gas
1Strong forces of attraction between particles:
2Weak forces of attraction between particles:
3Very weak forces of attraction between particles:
|
When a gas is heated, which of the following statements are true?
(Select all that apply)
If it's in a fixed container, the pressure will increase
If it's in an expandable container, the volume will decrease
If it's in a fixed container, the volume will increase
If it's in an expandable container, the volume will increase
|
In a closed system, changes in state won't change the mass at all.
Why is this the case?
The velocity of the particles remain the same
The bonds between the particles remain the same
The number of particles remain the same
|
Which state has the lowest density?
Liquid
Solid
Gas
|
"When heat is added, the particles move around faster and have enough energy to break the bonds altogether."
Which change of state does this describe?
Freezing
Boiling
Condensing
Melting
|
large / small / elastic / inelastic
Particle theory assumes all matter is made of , solid, and spheres
|
The particle model assumes that all particles are solid, ________ spheres.
inelastic
elastic
metal
|