Welcome to the Quiz!
This quiz contains 8 questions from a mix of 1 subtopics.
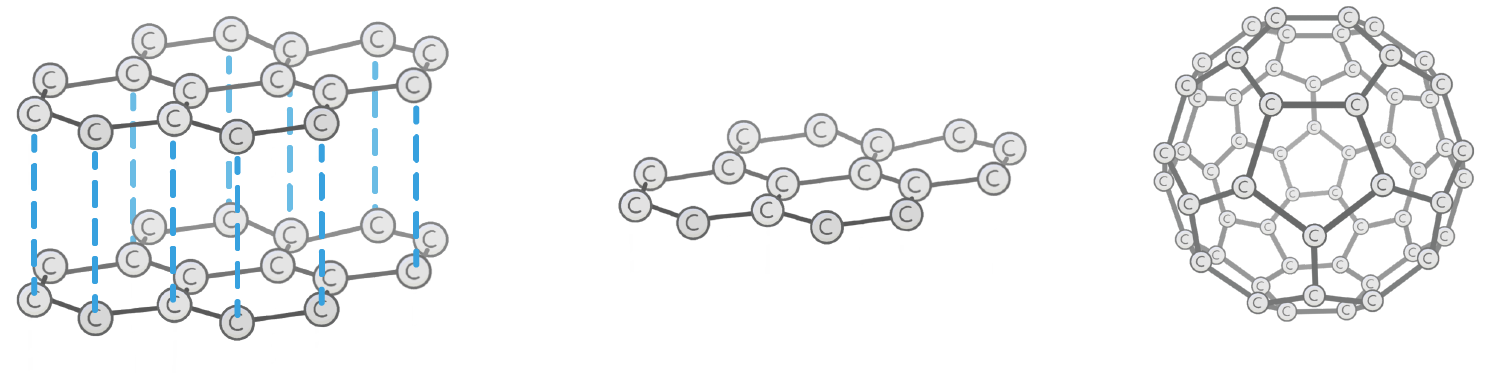
Graphite, graphene, and fullerenes, are all made from carbon in a solid state. Which word best describes these structures?
Isotopes
Alkanes
Allotropes
Isomers
|
conduct / delocalised / bonded / diamond / graphite
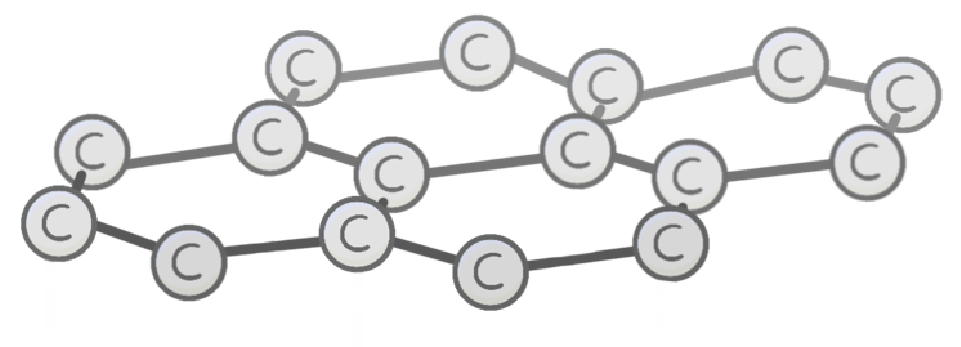
Graphene is just a single layer of , which consists of carbon atoms arranged into flat hexagons.
Each carbon has one electron, so graphene can electricity.
|
Graphene can be found naturally within graphite.
The sheets can be folded into two types of 3D structure: tubes and s, which are known as fullerenes.
|
F are allotropes of carbon, made by bending sheets of graphene into hollow structures.
Nanotubes made from these are useful in electronics. This is because each carbon atom has one delocalised which can carry charge.
|
Select two applications of fullerenes in industry:
Delivery of medicines around the body
Transport of food
Catalysts in chemical reactions
|
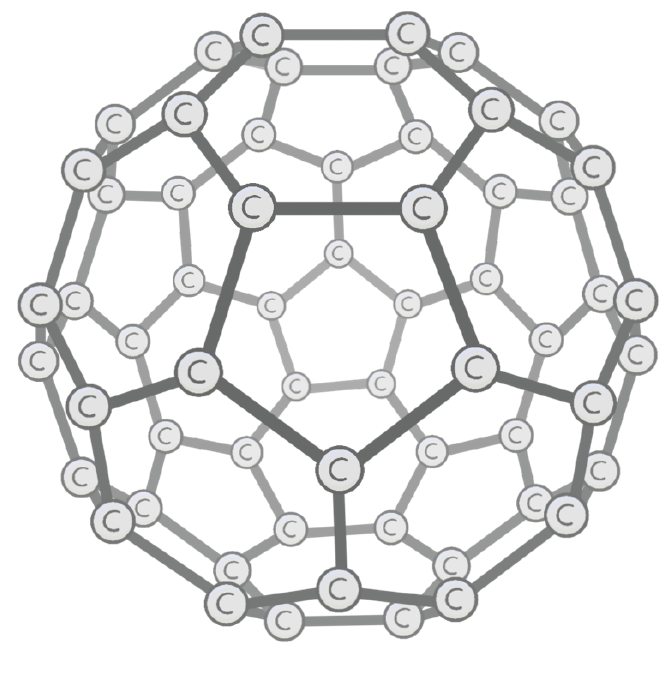
True or false? Fullerenes are useful catalysts in the chemical industry because they have a low surface area to volume ratio.
True
False
|
What is the formula of the first spherical fullerene produced, known as the Buckminster fullerene?
C20
C80
C40
C60
|
Which feature of carbon nanotubes makes them useful in tennis racket frames?
Their high surface area to volume ratio
Their ability to conduct electricity
Their high strength to weight ratio
|