Welcome to the Quiz!
This quiz contains 10 questions from a mix of 1 subtopics.
Two substances made from the same element, that are in the same physical state, but that have different structures, are called .
|
Select two allotropes of solid carbon from this list:
Graphite
Diamond
Silicone dioxide
Carbon dioxide
|
tiny / giant / lattice / cube / carbon / silicon
Diamond and graphite are examples of covalent molecular structures.
Both are made from the element arranged into a large regular repeating .
|
Which of the following statements are true of diamond?
Each carbon atom is bonded to 3 other carbon atoms
It is made up of silicon and oxygen
Each carbon atom is bonded to 4 other carbon atoms
It conducts electricity
|
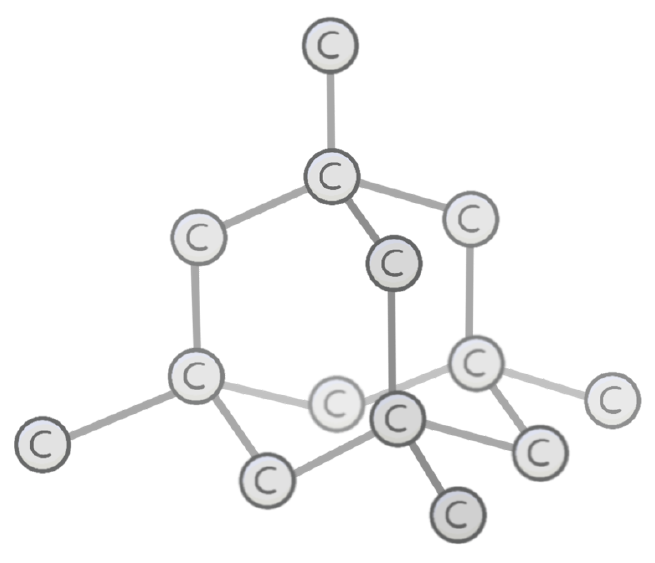
Which structure is shown in the image?
Carbon
Glass
Graphite
Diamond
|
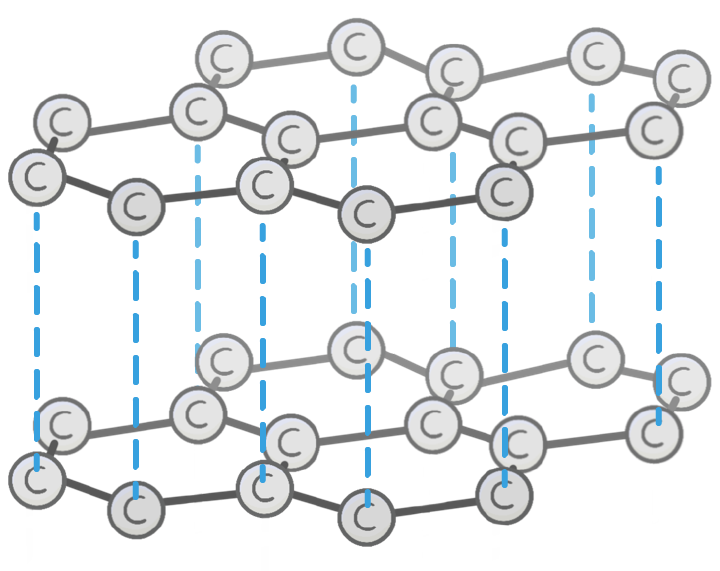
Which structure is shown in the image?
Graphite
Glass
Diamond
Carbon
|
In diamond, each atom is bonded to other atoms via covalent bonds.
This forms a regular 3D lattice which is very strong, and has very melting and boiling points.
|
Can diamond conduct electricity?
Yes
No
|
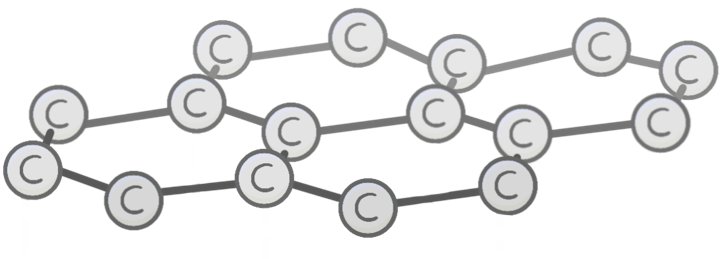
Unlike diamond, the carbon atoms in graphite each bond to other carbon atoms.
This arranges the graphite into 2D layers made up of repeating hexagons.
|
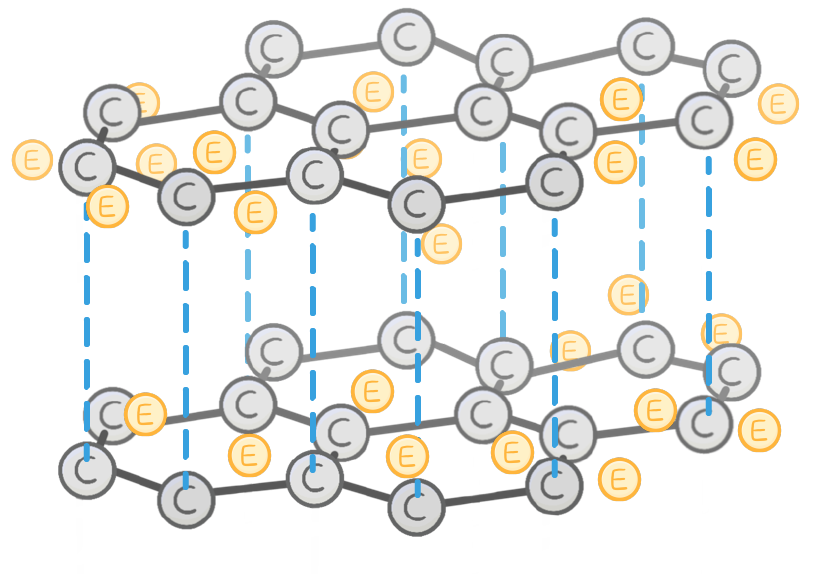
able / unable / localised / delocalised
Graphite is to conduct electricity.
This is because each carbon atom has one electron, which can move freely, and so is able to carry charge.
|