Welcome to the Quiz!
This quiz contains 8 questions from a mix of 1 subtopics.
covalent / ionic / simple / complex / small / giant
Atoms can share electrons to form bonds.
Covalent substances that only contain a few atoms are called molecular substances.
On the other hand, covalent substances with millions of atoms are called covalent structures.
|
Which of these is an example of a giant molecular structure?
Carbon dioxide
Ammonia
Water
Silicon dioxide (silica)
|
solid / gaseous / energy / intermolecular / high / low
In simple molecular substances, the individual molecules are held together by forces that exist between the molecules.
These intermolecular forces are weak and so don't require very much to break.
This means that they can be broken at relatively temperatures. As a result most simple molecular substances exist in the state at room temperature.
|
The halogens exist in different states at room temperature.
boiling / solid / liquid / gas / smaller / larger
- Chlorine is a at room temperature.
- Bromine is a at room temperature.
- Iodine is a at room temperature and gives off purple fumes.
The reason they are in different states at room temperature is that they have different melting and points. As you go down group 7, the atoms (and thus molecules) get . This means there will be more intermolecular forces, and so more energy (and a higher temperature) will be required to break them.
|
Select two properties of simple molecular substances:
Unable to conduct electricity
Low boiling points
High boiling points
Conduct electricity
|
small / giant / carbon / hydrogen / shape / lattice
Diamond and graphite are both made from the element , and are examples of covalent structures.
Their atoms are arranged in a regular repeating structure with many multiple covalent bonds between each atom.
|
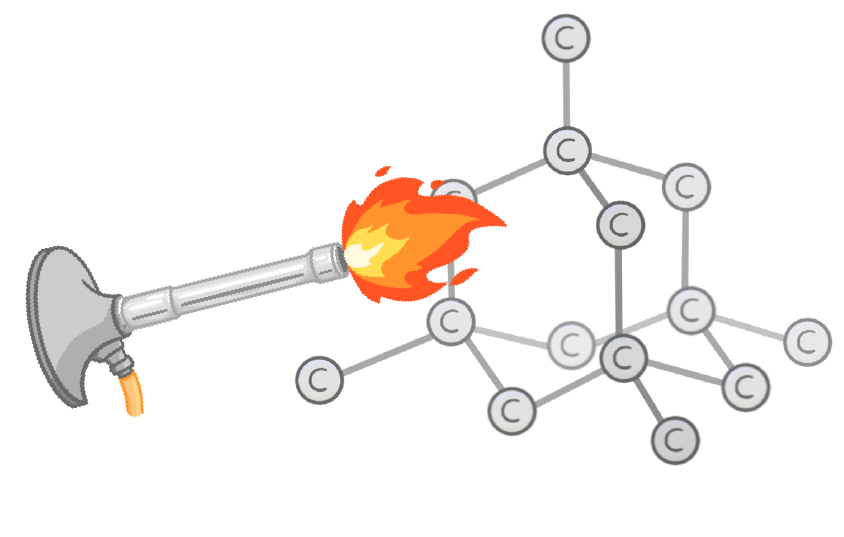
Do giant covalent structures have high or low melting points?
High
Low
|
Select all the properties of diamond.
(Select all that apply).
Conducts electricity
Low melting and boiling point
Weak
Strong
High melting and boiling point
Doesn't conduct electricity
|