Welcome to the Quiz!
This quiz contains 6 questions from a mix of 1 subtopics.
Electrons surround the atomic nucleus in regions called s.
The shell closest to the nucleus can hold electrons, whereas the outer ones can hold up to electrons.
|
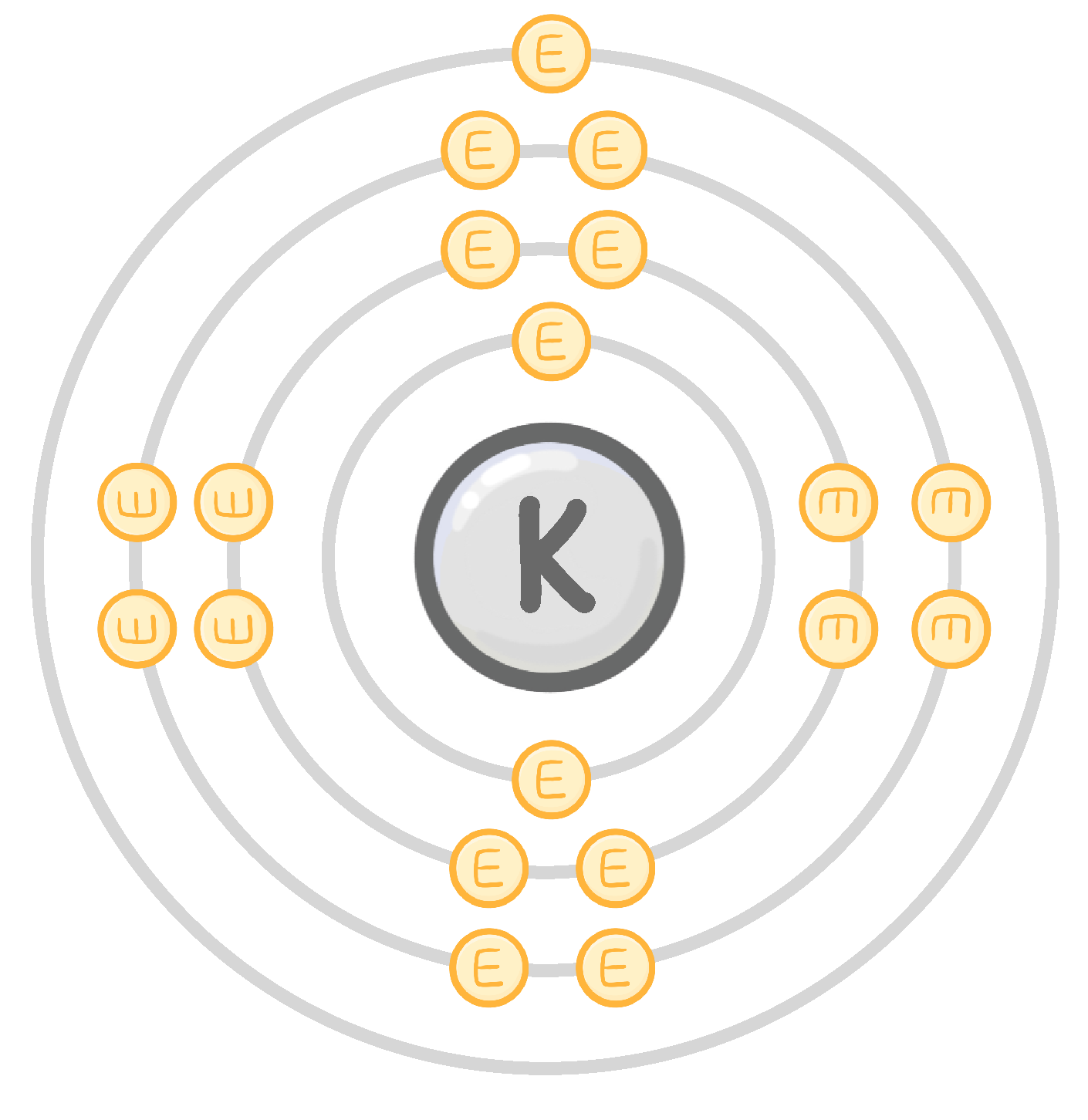
An atom of potassium has 1 electron in its outermost shell.
In a chemical reaction, will potassium gain or lose 1 electron?
Gain
Lose
|

Nitrogen has an atomic number of 7.
Which of the following is the correct electron configuration for an atom of nitrogen?
2, 5
3, 4
7
5, 2
|
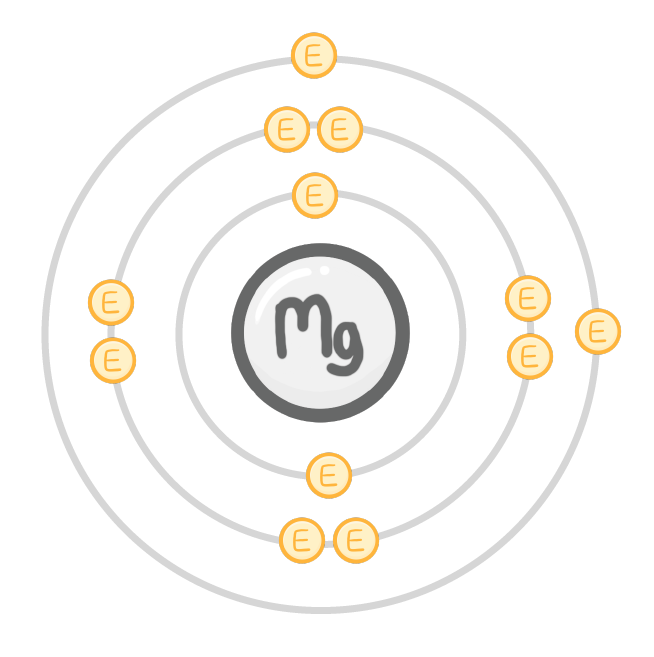
An atom of magnesium has the electron structure 2, 8, 2.
When a magnesium atom takes part in a reaction, the atom loses electrons.
The new electron structure is: , .
|
An atom of oxygen has 6 electrons in the outer shell. In a reaction, it gains 2 electrons.
What is the charge of the oxide ion created?
1+
2+
2-
1-
|