Welcome to the Quiz!
This quiz contains 10 questions from a mix of 1 subtopics.
The steps of simple distillation:
evaporates / gas / Bunsen / condenses
- A solution is heated, usually using a burner.
- The liquid in the mixture into a gas.
- The gas is cooled by a water jacket, and into a liquid, which then flows into the beaker.
|
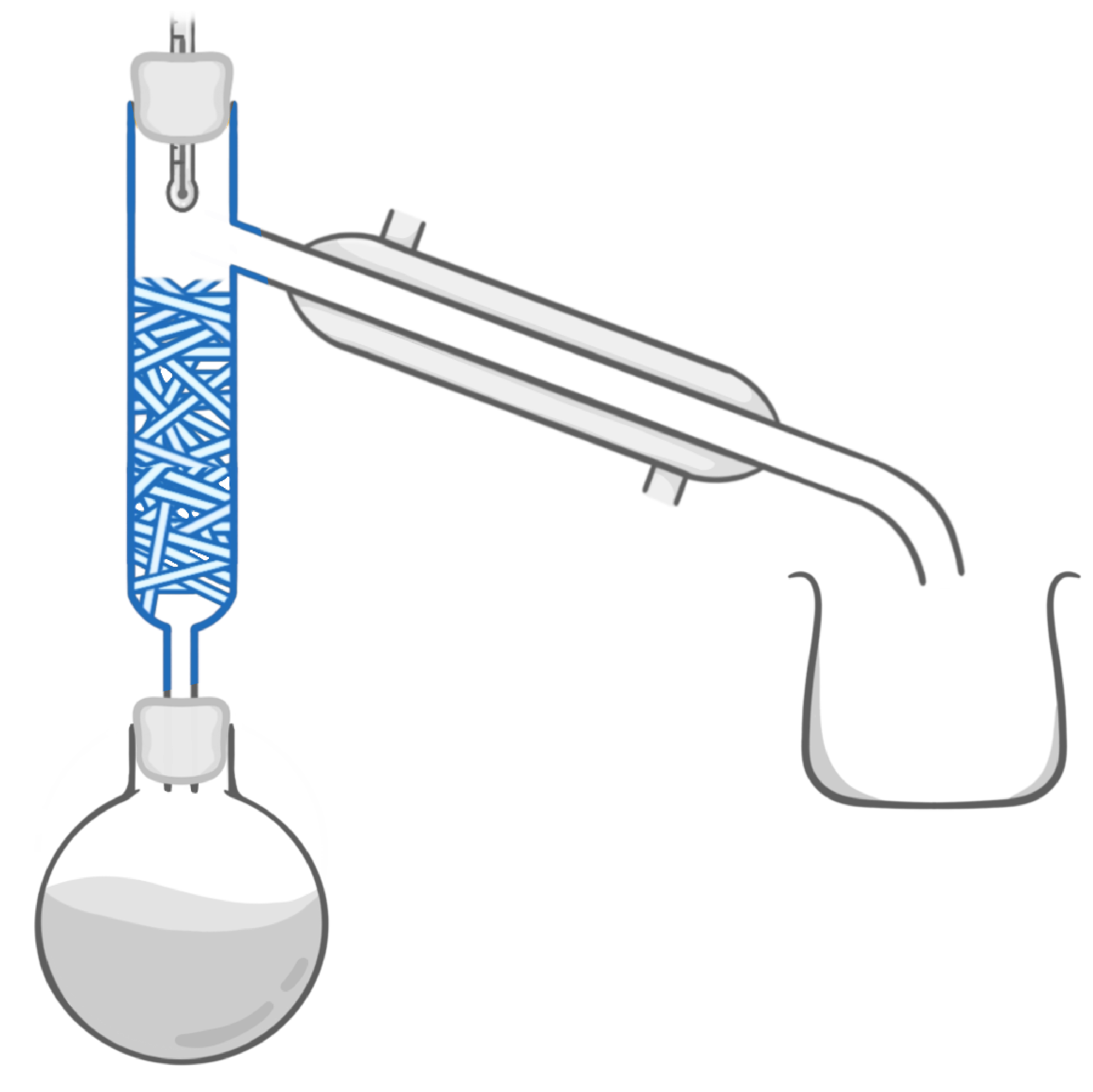
Which piece of equipment is highlighted in the image above?
Boiling flask
Fractionating column
Beaker
Cooling jacket
Condenser
|

Which piece of equipment is highlighted in the image above?
Condenser
Cooling jacket
Beaker
Fractionating column
Boiling flask
|
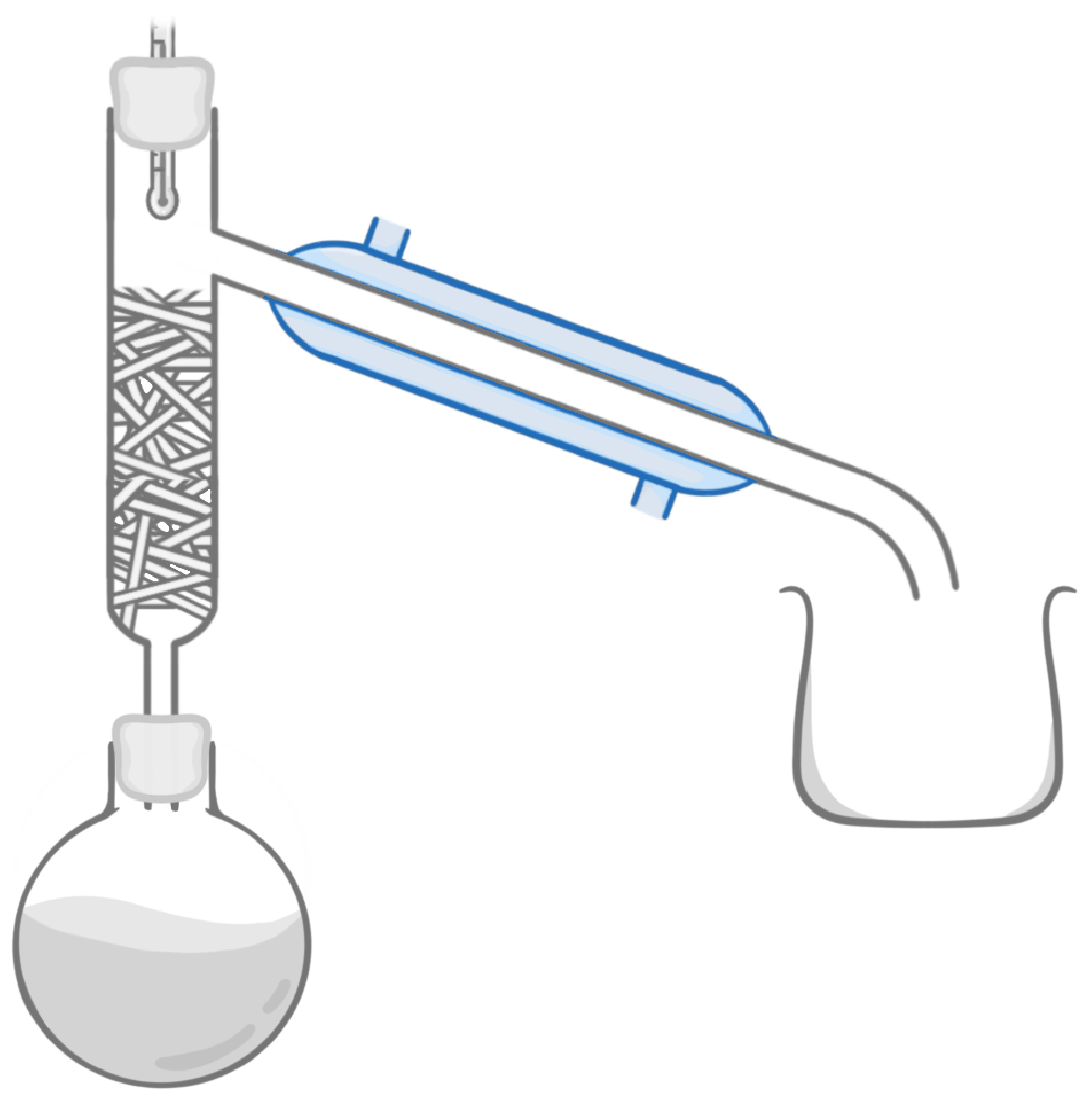
Which piece of equipment is highlighted in the image above?
Fractionating column
Beaker
Boiling flask
Cooling jacket
Condenser
|
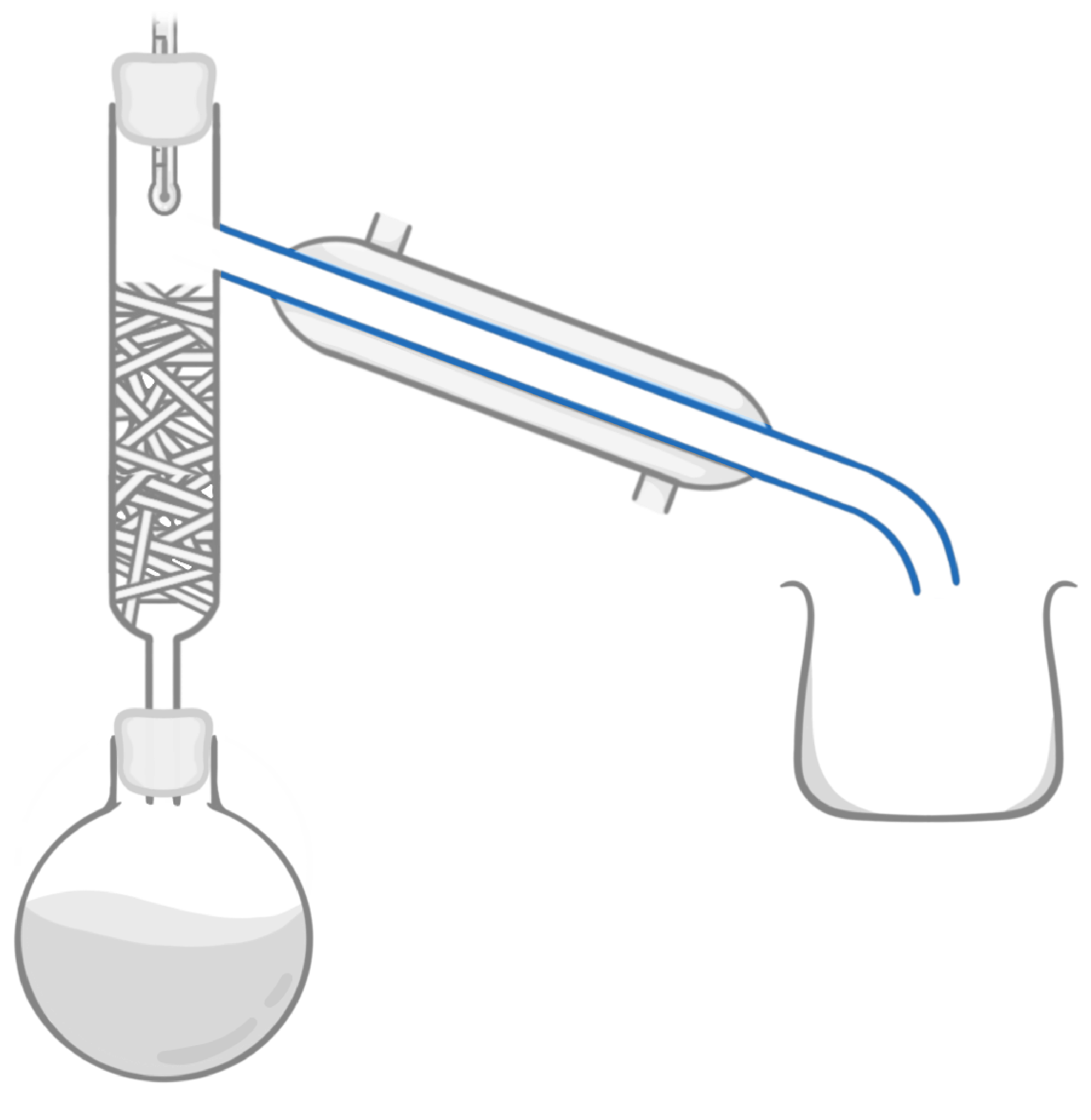
Which piece of equipment is highlighted in the image above?
Condenser
Fractionating column
Cooling jacket
Beaker
Boiling flask
|
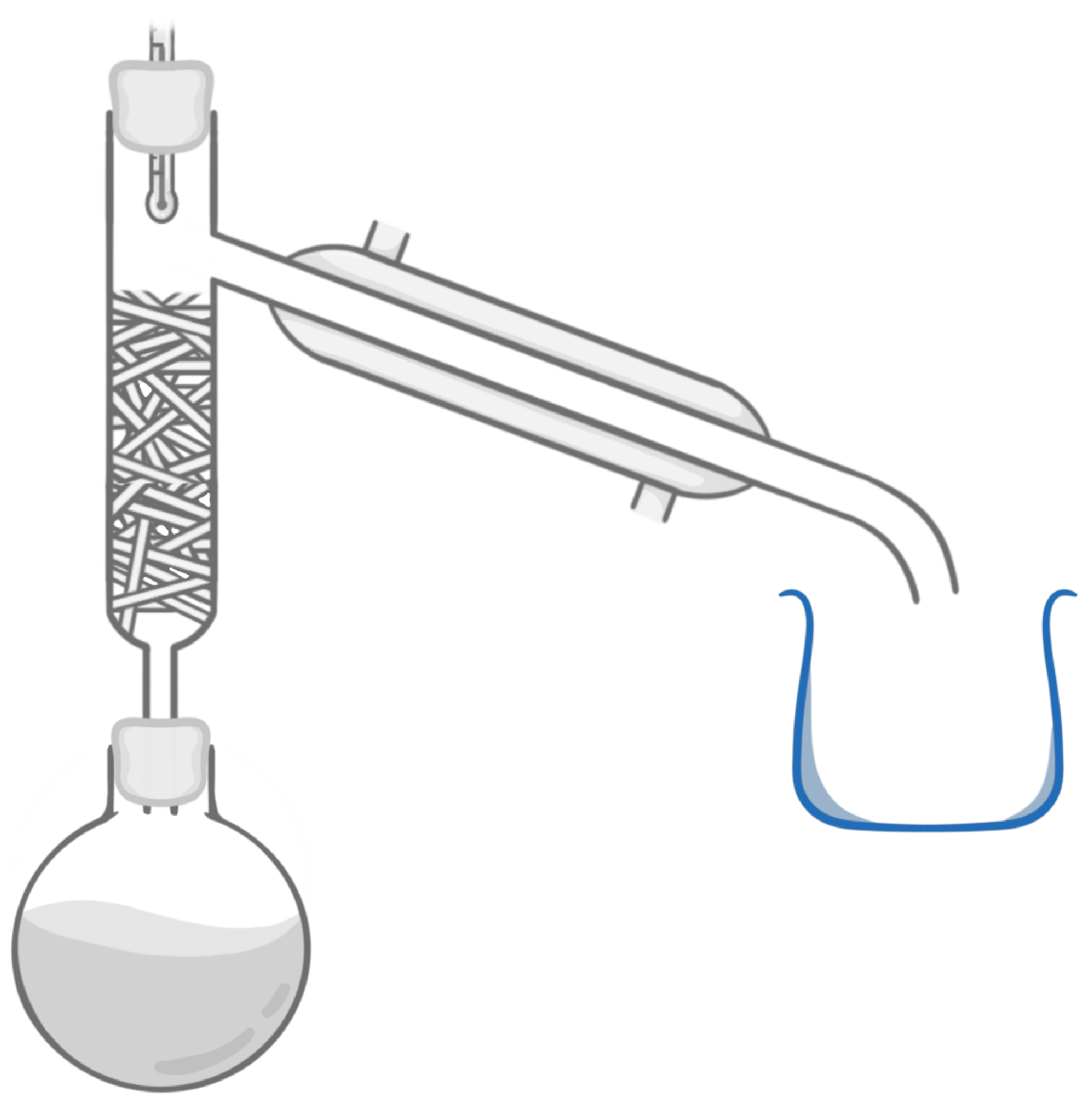
Which piece of equipment is highlighted in the image above?
Cooling jacket
Beaker
Condenser
Fractionating column
Boiling flask
|
Fractional distillation can separate a mixture of several liquids that each have different:
Melting points
Colours
Boiling points
|
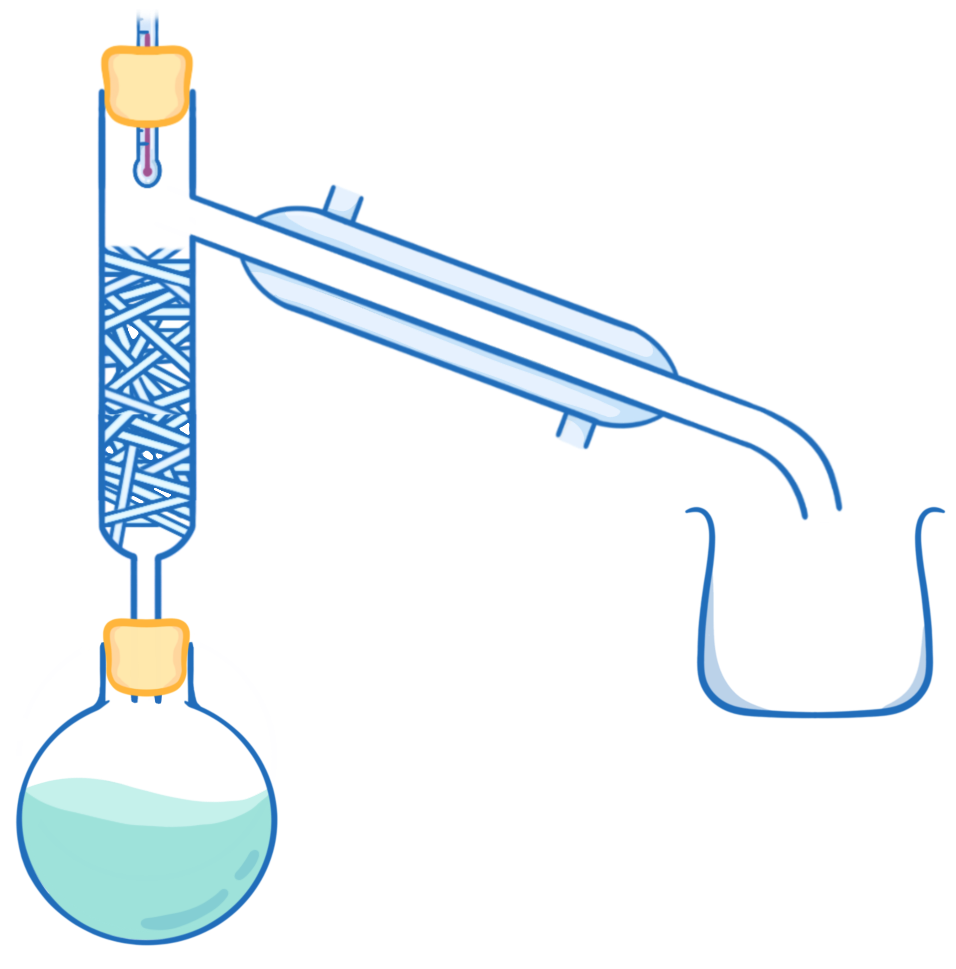
Which of the following is the correct order of steps required for fractional distillation?
Condenser ➔ beaker ➔ fractionating column ➔ boiling flask
Boiling flask ➔ fractionating column ➔ condenser ➔ beaker
Boiling flask ➔ condenser ➔ beaker ➔ fractionating column
|
Briefly explain the process of fractional distillation to separate a mixture of the liquids methanol, ethanol and propanol.
|
How would you isolate pure water from salt water?
Crystallisation
Fractional distillation
Simple distillation
Thermal decomposition
|