Welcome to the Quiz!
This quiz contains 8 questions from a mix of 1 subtopics.
tight / metallic / special / ionic / covalent
Metals can bind to non-metals via bonding, whereby particles with opposite charges are attracted to each other.
Non-metals can bind to other non-metals via bonding, whereby electrons are shared.
Metal atoms can bind to other metals using bonding.
|

Which of the following are properties of metals?
(Select all that apply)
High melting points
Unable to conduct electricity
High strength
Malleable
Low strength
|
positive / negative / localised / delocalised
In metallic bonding, each metal atom becomes an ion with a charge.
It does this by giving up its outer shell electrons.
These electrons are said to be '', and are shared across all the ions in the structure.
|
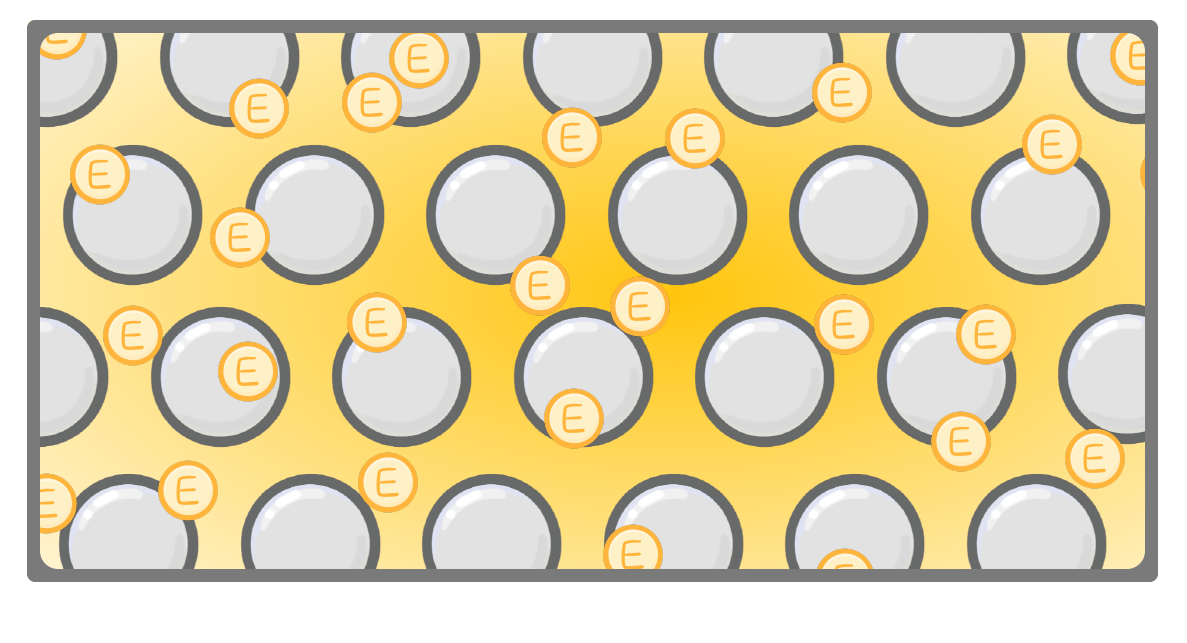
heat / electricity / thermal / chemical
In metallic bonding, electrons are delocalised from the lattice of metal ions.
These electrons can carry charge, and so metal acts as a conductor of .
These electrons can also carry energy, meaning metals are good conductors of heat.
|
Metals are malleable. What does this mean?
They are brittle and are easily cut
They are shiny in texture
They can be bent or hammered into shape
They conduct heat and electricity
|
Which of these describes an alloy?
A metal combined with one or more other elements
A non-metal combined with one or more other elements
A pure metal
|
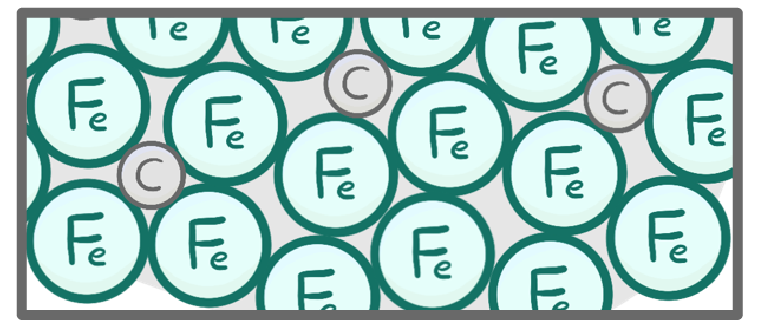
lower / higher / charges / sizes / slide / jump
Alloys tend to have a strength than pure metals.
This is because the atoms/ions of the different elements are different , which disrupts the regular layered structure and so means the layers can no longer over one another.
|
Which of these is an alloy?
Water
Vanadium
Iron
Steel
|