Welcome to the Quiz!
This quiz contains 10 questions from a mix of 1 subtopics.
Which of the following is the reaction of the Haber process?
nitrogen + oxygen ➔ nitrogen dioxide
nitrogen dioxide + hydrogen ➔ ammonia + water
nitrogen + hydrogen ➔ ammonia
|
Balance the the following equation:
N2 + H2 ⇌ NH3
|

Is the Haber process an exothermic or endothermic reaction?
Exothermic
Endothermic
|
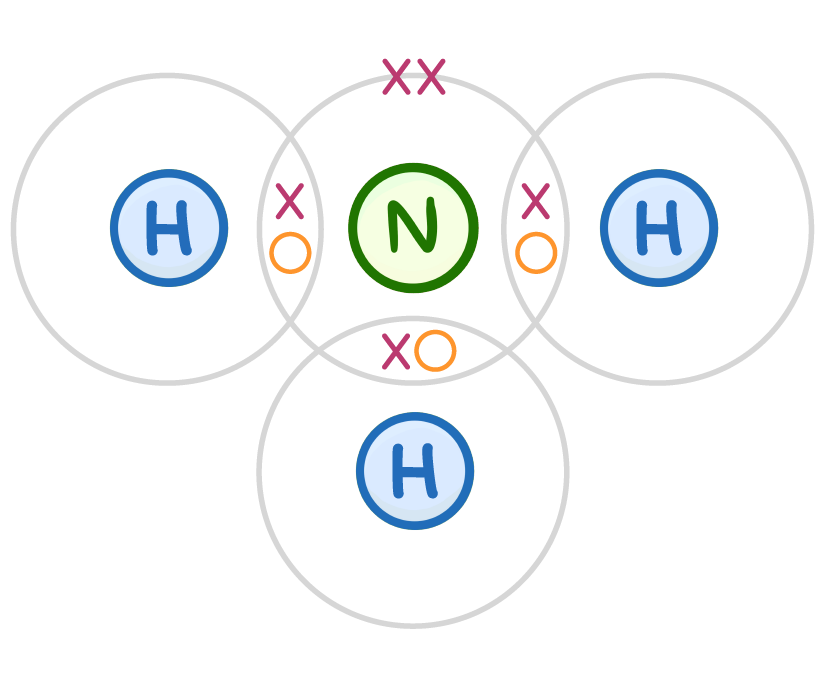
Why is ammonia so important?
It is used in medicines
It is used in fertilisers
It is used as a fuel
|
Which metal acts as a catalyst for the Haber process?
Iron
Aluminium
Nickel
|
Where do we get the nitrogen required for the Haber process from?
Take it from the air
Waste product of sewage treatment
Make it from hydrocarbons
|
Where do we get the hydrogen required for the Haber process from?
Take it from the air
Make it from hydrocarbons
Waste product of sewage treatment
|
What does the '⇌' symbol mean in a chemical equation?
There is not enough energy for the reaction to take place
The reaction is very fast
The reaction is reversible
|
Explain why a temperature of 450°C is used in the Haber process.
|
Explain why a pressure of 200 atmospheres is used in the Haber process.
|